K1
Cat.No.: CSC-C9201W
Species: Homo sapiens (Human)
Source: Thyroid Gland Metastasis
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
K1 is a human papillary thyroid carcinoma (PTC) cell line. It was isolated from a metastatic lesion of a thyroid carcinoma patient. K1 cell line has been one of the most extensively used in vitro model system for studies on the molecular pathogenesis, signaling, and therapeutics of papillary thyroid cancer, which is the most common type of thyroid cancer. The cells are epithelial with a cobblestone morphology, and form an adherent monolayer of cells. The cells retain thyroid specific differentiation markers such as thyroglobulin and wild type p53. Moreover, K1 cells maintain typical epithelial morphology and harbor many of the important genetic changes found in PTC including BRAFV600E mutation, which leads to constitutive activation of MAPK/ERK signaling. In culture, K1 cells show adherent growth and form tumor xenografts in immunodeficient mice.
Low-Dose Oxidants Reduce Metabolic Activity, Proliferation, and Viability in a Human Papillary Thyroid Carcinoma Cell Line
Medical gas plasmas are emerging in oncology, targeting tumor redox states with reactive species, akin to approved therapies. They are ideal for treating inaccessible internal tumors through intratumoral injection of oxidant-enriched liquids. Lens et al. evaluated the effects of plasma-oxidized DMEM (oxDMEM) on human papillary thyroid cancer cells (K1), assessing resistance, proliferative activity, and oxidative stress response.
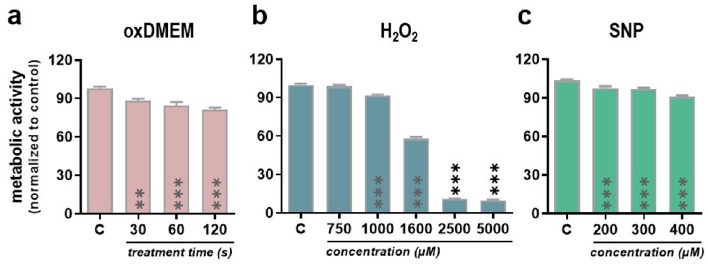
Cells were exposed to plasma-oxidized DMEM (oxDMEM), hydrogen peroxide (H2O2), and sodium nitroprusside (SNP, an RNS donor). The composition and quantity of ROS/RNS in plasma-oxidized liquids depend on factors like feed gas, energy supply, treatment time, and jet-to-target distance. In this study, the jet-to-liquid surface distance was 23.3 mm, and the jet was operated with argon at 3 slm. oxDMEM was generated by exposing DMEM to plasma for 30, 60, or 120 seconds and added to cells for 24 hours. oxDMEM significantly reduced the metabolic activity of K1 cells in a dose-dependent manner (Fig. 1a). Similar effects were seen with H2O2 (Fig. 1b) and SNP (Fig. 1c), though higher H2O2 concentrations (>1 mM) were needed for significant effects. The proliferative activity of K1 cells was also reduced by oxDMEM, H2O2, and SNP in a dose-dependent manner (Fig. 2a, b). Toxicity, assessed using trypan blue staining, was highest with oxDMEM and SNP and lowest with H2O2 (Fig. 2c).

APOE Expression in Papillary Thyroid Carcinoma: Influencing Tumor Progression and Macrophage Polarization
Metastatic papillary thyroid carcinoma (PTC) is challenging to treat, prompting research into immunotherapy. Tumor-associated macrophages (TAMs) influence tumor progression, and apolipoprotein E (APOE) can regulate macrophage polarization and tumor microenvironment remodeling. However, APOE's role in TAM polarization and biological functions in PTC is unclear. Huo's team investigated APOE's effects on TAM polarization and PTC progression.
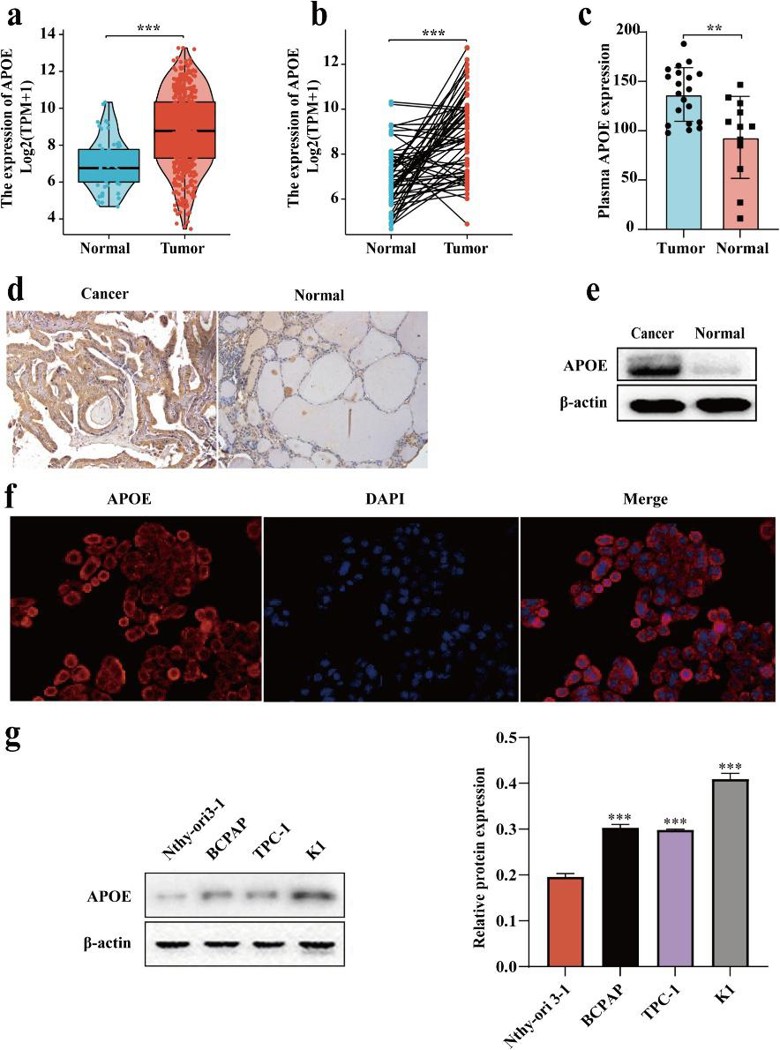
Analysis of the TCGA dataset, immunohistochemistry and western blot showed that APOE was highly expressed in PTC tissues (Fig. 3a-e). APOE was mainly found in the cytoplasm and along the plasma membrane (Fig. 3f). In PTC cell lines (K1, TPC-1, BCPAP), APOE expression was higher than in normal thyroid cells (Nthy-ori 3-1), with the highest levels in K1 cells (Fig. 3g). RT-qPCR showed that shAPOE fragments 1 and 3 effectively silenced APOE in K1 cells, so they were used in later experiments. To study APOE's role in PTC tumor initiation and lymph node metastasis, they used APOE-knockdown K1 cells. Growth curve and clone formation assays showed that APOE silencing reduced K1 cell proliferation (Fig. 4a, b). Flow cytometry indicated that APOE knockdown increased apoptosis and blocked the G1 to S phase transition (Fig. 4c). Western blot showed increased Caspase 3 and Bax, and decreased Cyclin D1 and Bcl-2 in shRNA#1#3 cells compared to shRNAC cells (Fig. 4d). In wound healing and transwell assays, APOE overexpression enhanced K1 cell migration and invasion.

Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells