
Human Intestinal Microvascular Endothelial Cells (HIMEC)
Cat.No.: CSC-7768W
Species: Human
Source: Intestine
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human intestinal microvascular endothelial cells (HIMEC) are isolated from microvasculature of intestinal mucosa and submucosa. The intestine is the largest and the most functionally diverse part of the human digestive system. HIMECs have been shown to express a variety of cell adhesion molecules, including ICAM-1, VCAM-1, and E-selectin, and chemokines, such as IL-8 and IL-12, and thereby play an important role in leukocyte adhesion and migration. In the context of inflammatory bowel disease (IBD), the adhesion capacity of HIMECs is greatly increased and inflammatory cells can be recruited to the site of inflammation. HIMECs have a role in the formation and maintenance of intestinal vasculature and are important to the pathogenesis of intestinal microenvironment in inflammation and tumors. HIMECs express CXCR4 and its ligand CXCL12 (SDF-1) and contribute to angiogenesis in the tumor microenvironment. The response of HIMECs to IL-8 also implicates them in the process of angiogenesis. HIMECs have been shown to display endotoxin tolerance with reduced cytokine expression and reactive oxygen species (ROS) production upon repeated stimulation by lipopolysaccharide (LPS), which is possibly related to the maintenance of intestinal immune homeostasis. HIMECs also express Toll-like receptor 5 (TLR5), which can recognize bacterial flagellin and thereby elicit an innate immune response during intestinal infection.
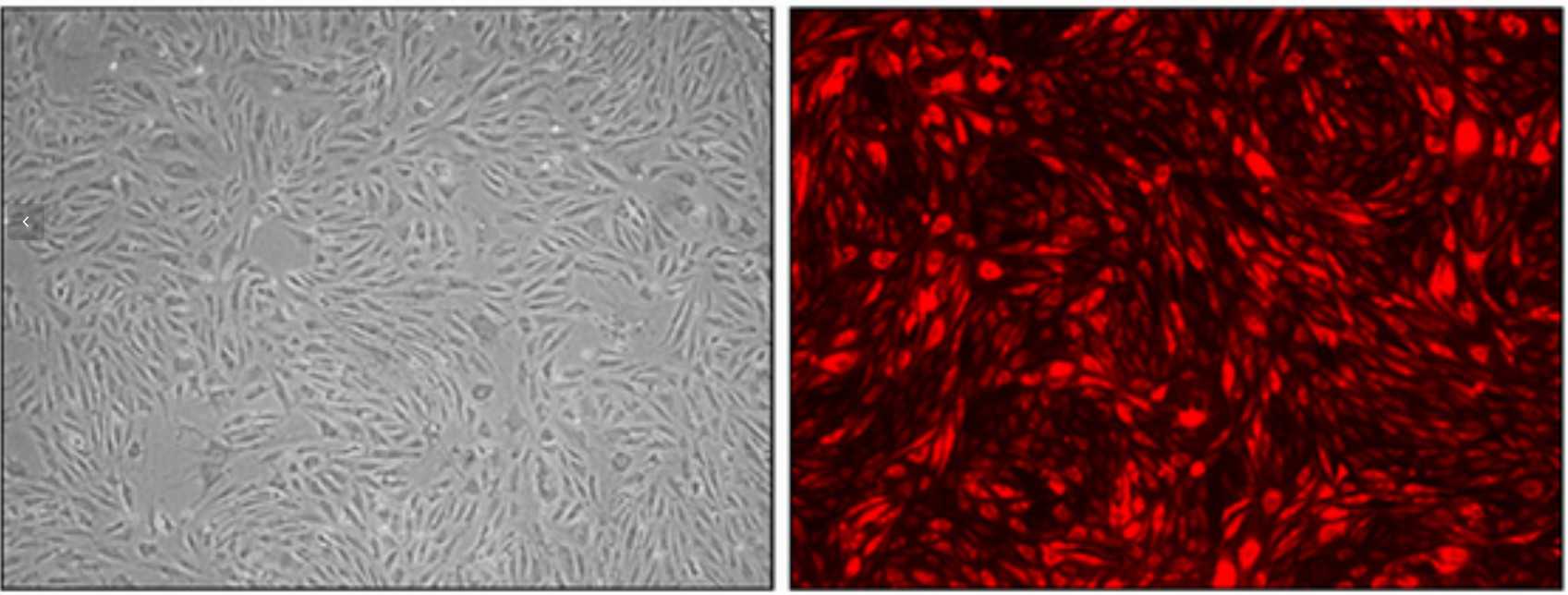 Fig. 1. Cell cultures of HIMEC at passage 10 that have been infected with lentiviral vectors containing a red fluorescent protein (Sordi V, Ferri A, et al., 2016).
Fig. 1. Cell cultures of HIMEC at passage 10 that have been infected with lentiviral vectors containing a red fluorescent protein (Sordi V, Ferri A, et al., 2016).
FA Treatment Restores Cell Viability and Inhibits TNF-α-Induced Cell Inflammation
Ulcerative colitis (UC) is a chronic inflammatory bowel disorder. Ferulic acid (FA), a traditional Chinese herb known for its antioxidant, anti-apoptotic, and anti-inflammatory properties, has an unclear role in UC. Yu et al. aimed to investigate FA's effects on UC.
In vivo, UC was induced in rats using 2,4,6-tribrobenzene sulfonic acid, followed by FA treatment. FA improved intestinal injury and reduced levels of inflammatory factors (IL-6, IL-12, IL-1β), apoptosis-related proteins (caspase-1, caspase-3), and the TXNIP/NLRP3 pathway in UC rats. To examine FA's effects on cell viability and inflammation, intestinal endothelial cells (HIMECs) were pretreated with various FA concentrations and TNF-α to induce damage. In control and solvent groups, cells grew normally, while TNF-α-treated cells showed reduced proliferation. FA treatment enhanced proliferation in a concentration-dependent manner (Fig. 1a). MTT assays indicated FA restored cell viability in TNF-α-treated HIMECs (Fig. 1b). ELISA results showed TNF-α increased IL-1β, IL-6, and IL-12, which FA subsequently reduced (Fig. 1c-e). These findings suggest FA improves cell viability and reduces TNF-α-induced inflammation.
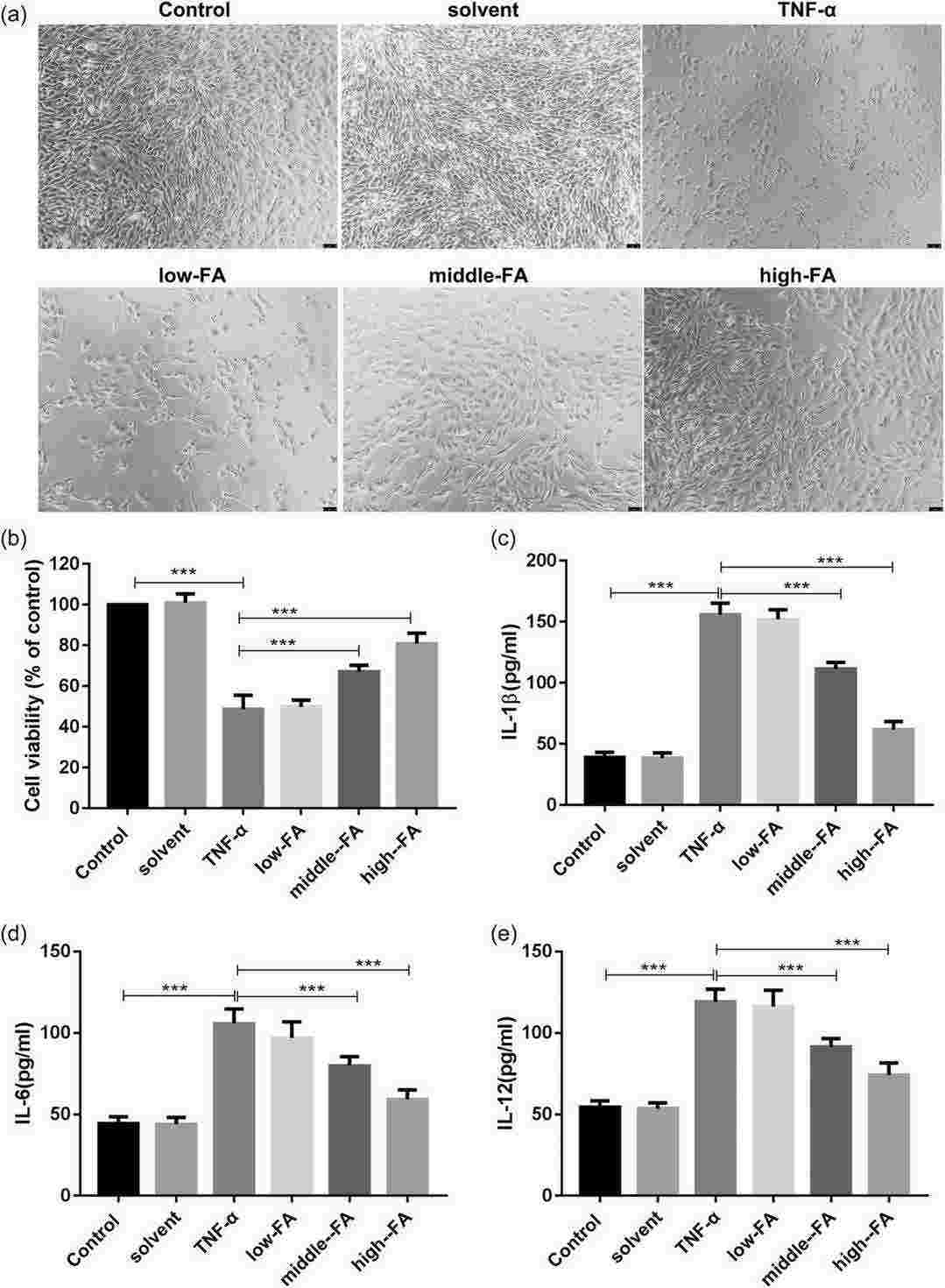 Fig. 1. FA treatment restores the viability of HIMECs and inhibits TNF-α-induced cell inflammation (Yu S, Qian H, et al., 2022).
Fig. 1. FA treatment restores the viability of HIMECs and inhibits TNF-α-induced cell inflammation (Yu S, Qian H, et al., 2022).
Plasmodium falciparum Parasite Lines Expressing DC8 and Group A PfEMP1 Bind to Brain, Intestinal, and Kidney Endothelial Cells
Cytoadhesion of Plasmodium falciparum-infected red blood cells cause microvascular blockages and organ issues. While the gastrointestinal tract acts as a primary sequestration site in fatal cerebral malaria, kidney complications are also seen in severe cases; however, detailed interactions at these sites are not well understood. Ortolan et al. studied parasite preferences for primary human microvascular endothelial cells from intestine (HIMEC) and kidney (HKMEC).
They examined CD36, ICAM-1, and EPCR expression on CD31+ human endothelial cells to determine parasite affinity for brain, gut, and kidney microvascular sites. Autopsies suggest higher parasite accumulation in peritubular capillaries versus glomerular ones. They compared endothelial cells from brain (HBMEC), intestine (HIMEC), and kidney (HKMEC) using flow cytometry, both under resting conditions and after TNF-α stimulation (Fig. 2). CD36 had low expression on HBMEC and HIMEC and was negligible on HKMEC, regardless of TNF-α stimulation (Fig. 3). EPCR was broadly expressed across HBMEC and HIMEC, slightly decreasing with TNF-α (Fig. 3). ICAM-1 showed low initial levels on cells but increased to nearly 100% after TNF-α treatment on all cell types (Fig. 3). HKMECs exhibited distinct receptor levels, likely due to their VEGF-enriched culture medium, which enhances ICAM-1 expression. Despite differences, intestinal and brain endothelial cells had similar receptor profiles.
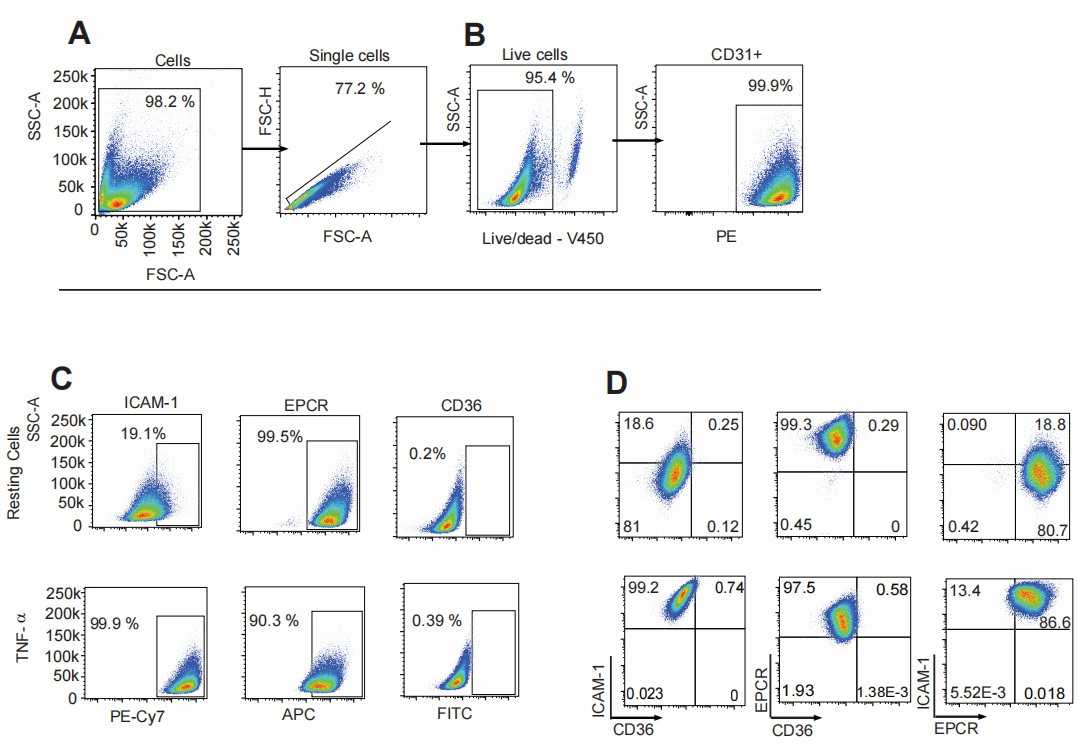 Fig. 2. Representative flow cytometry gate strategies used for analyzing primary endothelial cell populations (Ortolan L S, Avril M, et al., 2022).
Fig. 2. Representative flow cytometry gate strategies used for analyzing primary endothelial cell populations (Ortolan L S, Avril M, et al., 2022).
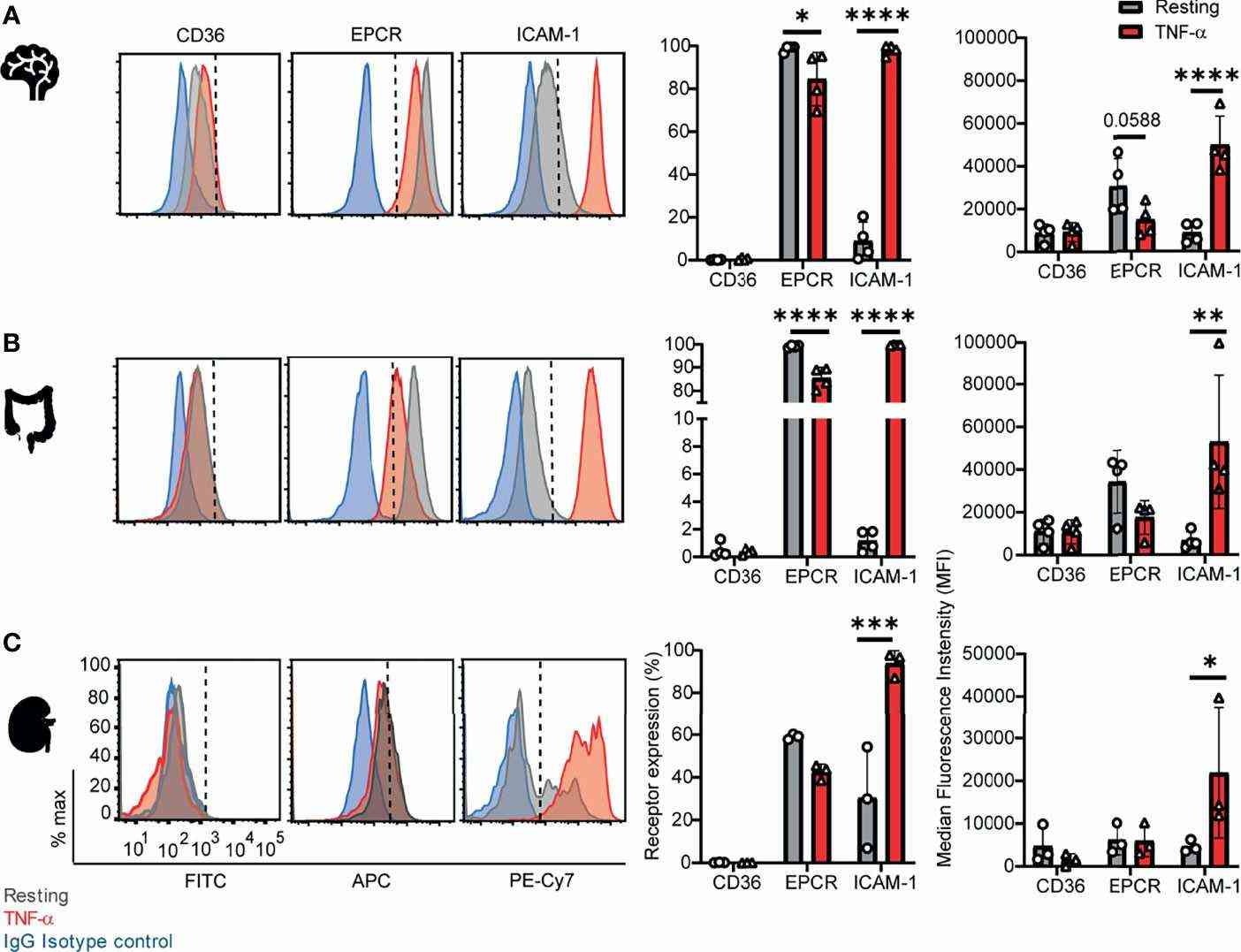 Fig. 3. Surface expression of CD36, EPCR, and ICAM-1 on primary human brain, intestinal and kidney endothelial cells (Ortolan L S, Avril M, et al., 2022).
Fig. 3. Surface expression of CD36, EPCR, and ICAM-1 on primary human brain, intestinal and kidney endothelial cells (Ortolan L S, Avril M, et al., 2022).
Ask a Question
Write your own review
