
Human Colonic Fibroblasts
Cat.No.: CSC-8242W
Species: Human
Source: Colon; Intestine
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
Human Colonic Fibroblasts (HCFs) are primary cells isolated from human colon tissue. These are not a single immortalized line but refer to a population of mesenchymal cells that are found in large abundance within the submucosa and muscular layers of the colon. In culture, HCFs maintain the characteristic spindle-shaped, elongated morphology that is adherent to the culture surface. They are responsible for synthesis and remodeling of the extracellular matrix (ECM) through production of collagen, fibronectin and other structural proteins. HCFs also secrete a variety of cytokines, chemokines and growth factors that help modulate local immune responses as well as epithelial cell behavior.
HCFs are commonly used for studies on inflammatory bowel disease (IBD), colorectal cancer, intestinal fibrosis and wound healing mechanisms. Research frequently utilizes these cells to examine cell interactions between epithelial structures and immune cells via tumor microenvironment co-culture models and stromal-epithelial communication studies. As with any primary cell, HCFs have a finite lifespan in culture, and may show some donor- and site-dependent variability. These limitations are generally offset by the high physiologic relevance of this model for studies of human-specific colonic pathophysiology and for screening of therapeutic agents with targets in fibrotic or inflammatory conditions.
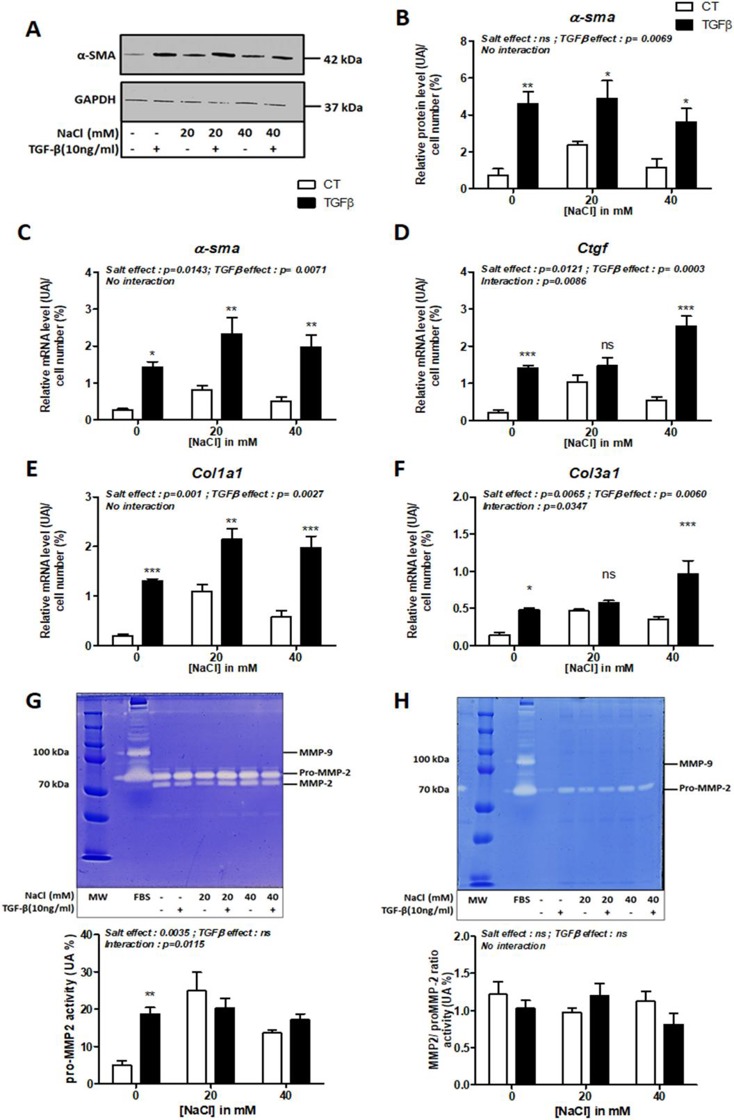
NaCl Treatment Promotes Factors Promoting IBD-Induced Fibrosis in Human Colonic Fibroblasts
Intestinal fibrosis is a common complication in inflammatory bowel diseases (IBD), resulting from the accumulation of extracellular matrix (ECM) proteins secreted by myofibroblasts. Identifying environmental factors, such as diet, that drive this risk is challenging. Diet, particularly high salt intake, appears to be a critical component in the development of intestinal fibrosis. Amamous et al. investigated the role of dietary salt in exacerbating intestinal fibrosis in a rat model of chronic TNBS colitis and in human colon fibroblast cells.
To test whether NaCl aggravates IBD-associated fibrosis by activating myofibroblast progenitors, they first tested the effect of NaCl on primary human colonic fibroblasts (CCD-18Co) treated with TGF-β. TGF-β significantly increased fibrosis markers such as α-SMA at mRNA and protein levels (Fig. 1A-C) and mRNA levels for Ctgf (p = 0.0003), Col1a1 (p = 0.0027), and Col3a1 (p = 0.0060) (Fig. 1D,E,F). NaCl treatment significantly increased mRNA levels of α-sma, Ctgf, Col1a1 and Col3a1 (Fig. 1C-F). There was a significant interaction between salt and TGF-β in the effect on Ctgf (p = 0.0086) and Col3a1 (p = 0.0347) mRNA expression (Fig. 1D, F). Gelatin zymography revealed that TGF-β or salt treatment did not alter pro-MMP-2 or MMP-2 activity in cell supernatants (Fig. 1G), and MMP-9 activity was undetectable in cell lysates (Fig. 1H). However, TGF-β upregulated pro-MMP2 activity in the absence of NaCl (Fig. 1G). NaCl treatment significantly affected pro-MMP2 activity (p = 0.0035, Fig. 1H).
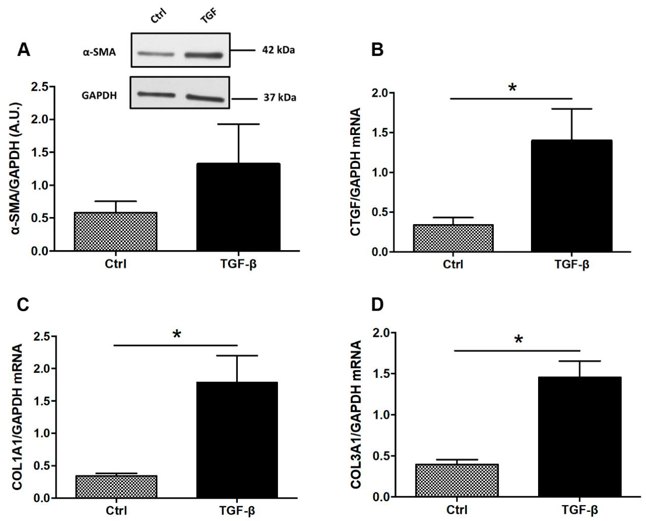
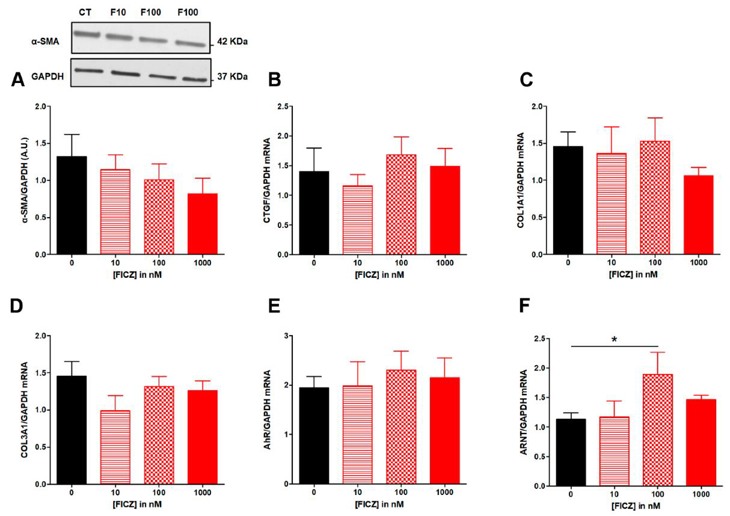
Dietary AhR Ligands Have No Anti-Fibrotic Properties in TGF-β1-Stimulated Human Colonic Fibroblasts
Intestinal fibrosis is a common complication in inflammatory bowel disease (IBD) without specific treatment. Aryl hydrocarbon receptor (AhR) activation is linked to improved clinical outcomes in models of intestinal inflammation. Amamous et al. aim to screen dietary AhR ligands for their anti-fibrotic properties in TGF-β1-stimulated human colonic fibroblast cells.
To evaluate the antifibrotic effects of tryptophan metabolites and curcumin in vitro, human colonic myofibroblasts (CCD-18Co) were TGF-β stimulated into a fibrotic phenotype. TGF-β drove expression of profibrotic genes such as COL1A1, COL3A1, and CTGF (Fig. 2B–D). TGF-β was tested on 3 intestinal epithelial cell lines (HT-29, HCT-8, and Caco-2) for 24, 48 and 72 hours. However, α-SMA expression was not detected in any of the epithelial cell lines. Additionally, in Caco-2 cells, TGF-β did not regulate expression of COL1A1 or CTGF. The authors then tested if treatment with AhR agonists repressed expression of ECM-associated proteins. FICZ (10–1000 nM) did not inhibit TGF-β induced α-SMA protein expression (Fig. 3A) or fibrotic gene expression (CTGF, COL1A1, COL3A1; Fig. 3B–D). AhR translocates to the nucleus and heterodimerizes with ARNT upon ligand binding, we assessed mRNA levels for AhR and ARNT. FICZ (10–1000 nM) did not regulate AhR mRNA levels (Fig. 3E), but 100 nM FICZ induced ARNT mRNA levels (Fig. 3F).
Ask a Question
Write your own review
