BJ 5ta
Cat.No.: CSC-C5144X
Species: Homo sapiens (Human)
Source: Skin; Foreskin
Morphology: Fibroblast-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
4 parts of Dulbecco's Modified Eagle's Medium containing 4 mM L-glutamine, 4.5 g/L glucose and
1.5 g/L sodium bicarbonate
1 part of Medium 199 Supplemented with 0.01 mg/ml hygromycin B
High-throughput screening
Toxicology
The BJ1-hTERT cell line is a genetically engineered, telomerase-immortalized derivative of the BJ strain of human foreskin fibroblasts. This model was created through the stable introduction of the human telomerase reverse transcriptase (hTERT) gene, which endows the cells with the ability to maintain telomere length, thereby bypassing replicative senescence and conferring an indefinite, yet stable, proliferative lifespan in vitro. Critically, this hTERT-mediated immortalization is considered non-transforming, as it does not typically disrupt core tumor suppressor pathways or confer anchorage-independent growth. Consequently, BJ1-hTERT cells retain essential characteristics of normal human diploid fibroblasts, including normal cell cycle checkpoints, contact inhibition, a stable karyotype, and a non-tumorigenic phenotype. This makes them a powerful and standardized alternative to primary fibroblasts, which suffer from limited passage capacity, donor-to-donor variability, and eventual senescence in culture.
Mitochondrial DNA Copy Number and Damage Following Exposure to X-rays in HeLa Cells and BJ1-hTERT Cells
This study investigated the radiation-induced impacts on mitochondrial DNA in vitro and in vivo, as well as the transgenerational inheritance.
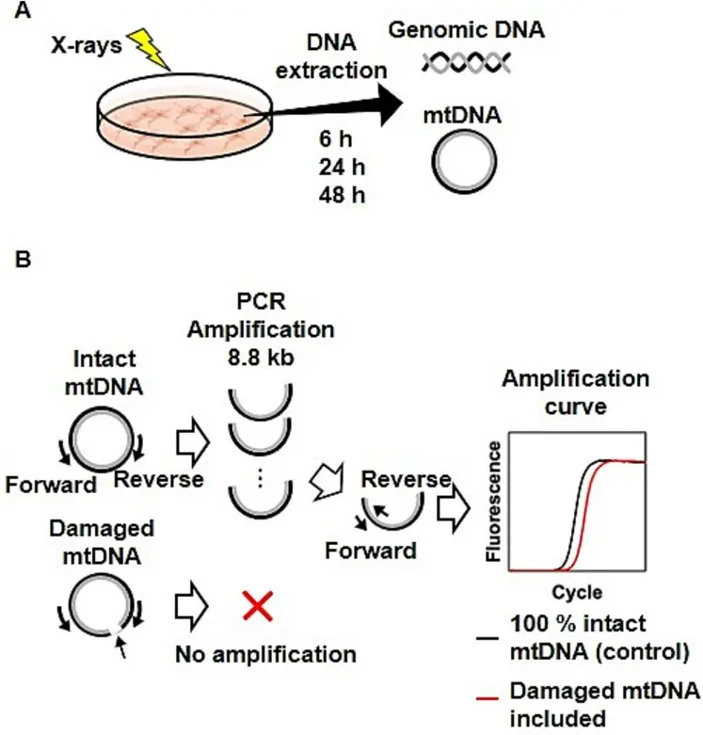
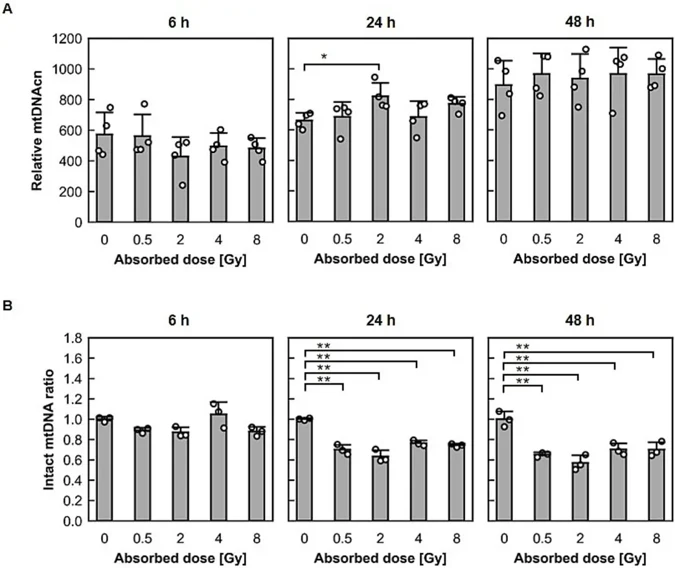
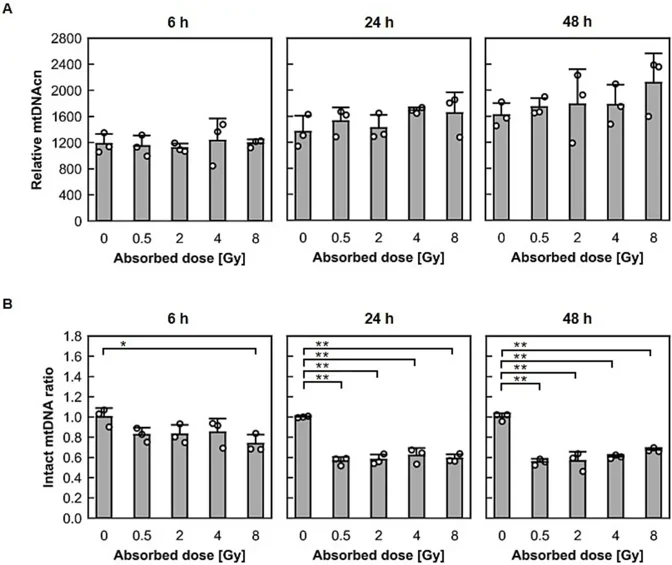
Human cervical cancer HeLa cells and telomerase-immortalized normal fibroblast BJ1-hTERT cells were exposed to X-rays at 0.5-8 Gy. As shown in Fig. 1A, DNA was extracted from HeLa and BJ1-hTERT cells cultured for 6, 24, and 48 h after irradiation. The mitochondrial DNA copy numbers (mtDNAcns) and radiation-induced damage were then examined by real-time PCR analysis (Fig. 1B).
As shown in Fig. 2A for HeLa cells and Fig. 3A for BJ1-hTERT cells, regardless of the dose, the mtDNAcn increased as the post-irradiation incubation time increased in both cell types. In HeLa cells, a significant increase in mtDNAcn was observed in 2-Gy-irradiated cells compared with nonirradiated cells 24 h after irradiation. As shown in Fig. 2B for HeLa cells and Fig. 3B for BJ1-hTERT cells, X-ray exposure changed the heteroplasmy of mtDNA. At 6 h after irradiation, the undamaged mtDNA ratio for BJ1-hTERT cells was significantly decreased in the 8-Gy-irradiated cells compared with that for control cells. At 24 h after irradiation, the intact mtDNA ratio for HeLa and BJ1-hTERT cells was significantly decreased in the irradiated cells compared with that for control cells. This trend remained 48 h after irradiation. Thus, X-ray exposure increased mtDNAcn in human cancer and noncancerous cells, regardless of the dose, and decreased the intact copy ratio, resulting in a dynamic shift of heteroplasmy.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells