STE-137
Cat.No.: CSC-C9061H
Species: Oncorhynchus mykiss (Rainbow trout)
Source: Embryo
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The STE‑137 cell line was first derived in 1963 from embryonic tissue of steelhead trout (Salmo gairdneri, now Oncorhynchus mykiss), and the line has been continuously maintained since, with greater than 250 passages. The line is very stable, and can be cryopreserved in liquid nitrogen for at least 14 years with no loss of phenotype.
STE 137 is one of the most commonly used salmonid cell lines in virology. It supports replication of several fish viruses, most notably infectious pancreatic necrosis virus (IPNV). Both lytic and persistent infections have been described. In the persistent state, STE 137 releases infectious virus at a steady titre while retaining normal morphology and being resistant to super infection with homologous IPNV strains. Electron microscopy revealed defective interfering (DI) particles of ~55 nm diameter in persistently infected cultures, indicating a role for DI viruses in establishing carrier states.
STE‑137 is also susceptible to a range of heterologous viruses including herpesvirus salmonis, chum salmon virus, and infectious haematopoietic necrosis virus, but with lower plaque efficiency. The line has been used for toxicological screening, for example to evaluate the impact of triphenyl phosphate on embryonic fish cells. STE‑137 is easy to obtain (through repositories such as the European Collection of Authenticated Cell Cultures), has a well-characterised growth profile, and is an extremely useful tool for research into fish diseases, vaccine development and environmental toxicology.
The Flame Retardant Triphenyl Phosphate Affects Cell Viability and Proliferation of STE-137 and Rtgill-W1
Triphenyl phosphate (TPhP), a common organophosphate flame retardant and plasticizer, is a ubiquitous pollutant that disrupts metabolic and estrogenic signaling and is considered an endocrine-disrupting chemical. Endocrine disruptors can remodel the epigenome, especially during embryogenesis. Here, Germain et al. asked whether environmentally relevant TPhP exposure alters the epigenome of two rainbow trout (Oncorhynchus mykiss) immortalized lines, STE-137 and RTgill-W1.
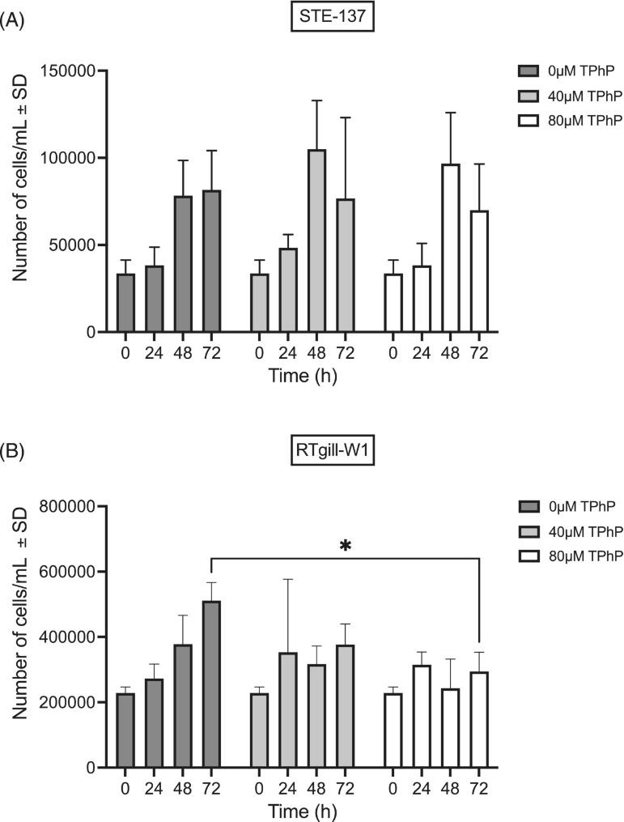
They determined a sub-lethal TPhP exposure regimen for epigenetic investigations in STE-137 and RTgill-W1 cells to the flame retardant triphenyl phosphate. They found that the 24 h LC50 for TPhP was 307 μM in STE-137 and 107 μM in RTgill-W1 cells (Fig. 1A, B). They determined that at 40 and 80 μM of TPhP, there was no significant difference in cell proliferation at 24 or 48 h post-exposure compared with control (Fig. 2A, B). However, following 72 h of exposure, 80 μM of TPhP caused a significant reduction in cell proliferation in RTgill-W1 cells (Fig. 2B). No significant differences in cell proliferation were found at any concentration at any time point compared with control (Fig. 2A).

Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells