RH-35
Cat.No.: CSC-C9240W
Species: Rattus norvegicus (Rat)
Source: Liver
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
RH-35 is an immortalized, rodent hepatocellular carcinoma (HCC) cell line with well-documented morphological, functional, and molecular characteristics. It was isolated from liver tumor tissue of a chemically induced male Fisher 344 rat (e.g., N-nitrosodiethylamine, DEN). The parental tumor is derived from hepatic parenchyma and recapitulates the pathology of moderately differentiated human HCC, the most prevalent clinical subtype of liver cancer. RH-35 cells have an epithelial-like phenotype when grown in vitro: polygonal or irregular with distinct borders, high nuclear/cytoplasmic (N/C) ratio (≈1:2), prominent nucleoli, and some binucleated cells. It grows as an adherent culture in high-glucose DMEM medium with 10% fetal bovine serum. It has a 36-48-hour doubling time and can be passaged stably for up to 50 generations.
RH-35 is widely used in liver research as a model to study the pathogenesis of HCC (e.g., chemical carcinogenesis, metabolic reprogramming), screen for hepatotoxic drugs (using LDH release or apoptosis assays), and test anti-HCC therapeutics (e.g., PI3K/ERK inhibitors). It is also used to help understand normal hepatic physiology in a comparative setting with normal rat hepatocytes (e.g., BRL-3A cells).
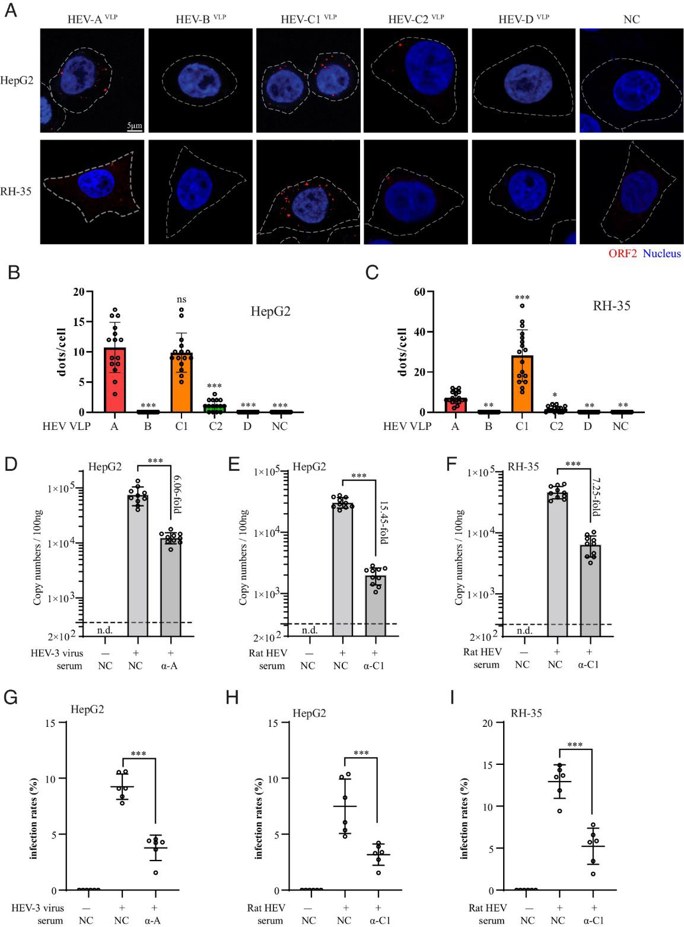
HEV-C1VLP and Rat HEV Complete the Entry Step and Migrate into Human Target Cells.
Classically, HEV variants causing human infection belong to HEV-A. However, the increasing cases of rat HEV infection in humans since 2018 challenged this notion. Guo's team investigated the cell binding tropism of rat HEV and compared it with other HEV species using virus-like particles (HEVVLPs)and infectious particles. They elucidated the mechanisms of zoonotic transmission and cross-protection among different HEV species.
Upon specific binding to the receptor(s), virions need to penetrate the cell membrane and enter the target cells to complete the so-called virus entry process. Herein, they inoculated HepG2 and RH-35 cells with the same amounts of VLPs. Consistent with their binding tropism, they detected marginal signal of HEV-C2VLP within HepG2 and RH-35 cells, and no HEV-BVLP and HEV-DVLP signals (Fig. 1A-C). Nevertheless, significant amounts of HEV-C1VLP and HEV-AVLP were observed within HepG2 and RH-35 cells (Fig. 1A-C, column 1 and 3). Furthermore, in parallel with the entry of human HEV-3 in HepG2 cells, rat HEV entered HepG2 and RH-35 cells. More convincingly, both RT-qPCR and immunofluorescent analysis indicated that the entry of rat HEV into HepG2 and RH-35 cells was significantly inhibited by anti-HEV-C1VLP serum (Fig. 1D-I). Collectively, these results demonstrated that HEV-C1VLP and infectious rat HEV particles possess the capability to enter human target cells.
Tumor Targeted Assay of an Autologous Erythrocyte-Anchoring Strategy with a Closed-System Drug-Transfer Device
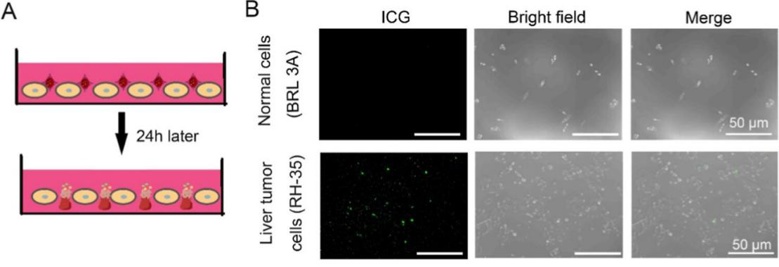
Chemotherapeutic agents are known to exhibit limited capacity for efficient localization to tumor sites when they are administered intravenously. This leads to off-target effects and poor accumulation in the desired target regions. Feng's team therefore designed an autologous ER-anchoring delivery strategy to potentiate the effects of chemotherapy drugs and mitigate adverse effects. A closed-system drug-transfer device was constructed for autologous ER procurement and immunogenicity attenuation. Doxorubicin (DOX) and indocyanine green (ICG) were encapsulated in autologous ERs and subsequently modified with DSPE-PEG-FA. The resulting DOX-ICG@ER-D was reinfused into circulation for the purpose of chemotherapy potentiation. In order to confirm that DOX-ICG@ER-D was capable of selectively accumulating within tumor cells, a coculture experiment was performed (Fig. 2A). At 24 h post-culture, they observed scant fluorescent DOX-ICG@ER-D localized to normal liver cells and bright fluorescence from liver tumor cells (RH-35) (Fig. 2B). Taken together, these data indicated that DOX-ICG@ER-D had a tumor-targeting capacity.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells