MOR/CPR
Cat.No.: CSC-C9512J
Species: Homo sapiens (Human)
Source: Lung
Culture Properties: Aggregates in suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
The MOR/CPR cell line is a cisplatin-resistant variant of MOR cells that has been subjected to long-term treatment with incrementally increasing concentrations of cisplatin. This prolonged selection pressure produces a stable chemoresistant phenotype that closely models clinically relevant mechanisms of platinum resistance in cancer cells. MOR/CPR has been shown to exhibit a significant decrease in cisplatin accumulation when compared to the parental MOR line, making it a model in which decreased intracellular uptake is a major contributor to chemoresistance. Modifications in membrane transport, metal-ion homeostasis, or transporter expression are all often associated with cisplatin resistance, making MOR/CPR a cell line of choice for studying uptake-limited resistance mechanisms.
In addition to being highly resistant to cisplatin, MOR/CPR also exhibits significant cross-resistance to the alkylating agent melphalan, implicating potential overlap in the mechanisms of the two agents, potentially including aspects of DNA damage tolerance or repair. Cells exhibit minimal or no cross-resistance to other platinum agents such as carboplatin or oxaliplatin, further emphasizing the unique nature of the acquired resistance and usefulness of this model in mechanistic or comparative drug-response studies.
As such, MOR/CPR is frequently used as a model for studying DNA repair, membrane transport, and molecular mechanisms of chemoresistance. It is used for testing and screening of chemosensitizers, understanding and reversal of chemoresistance, and development of second-generation platinum drugs.
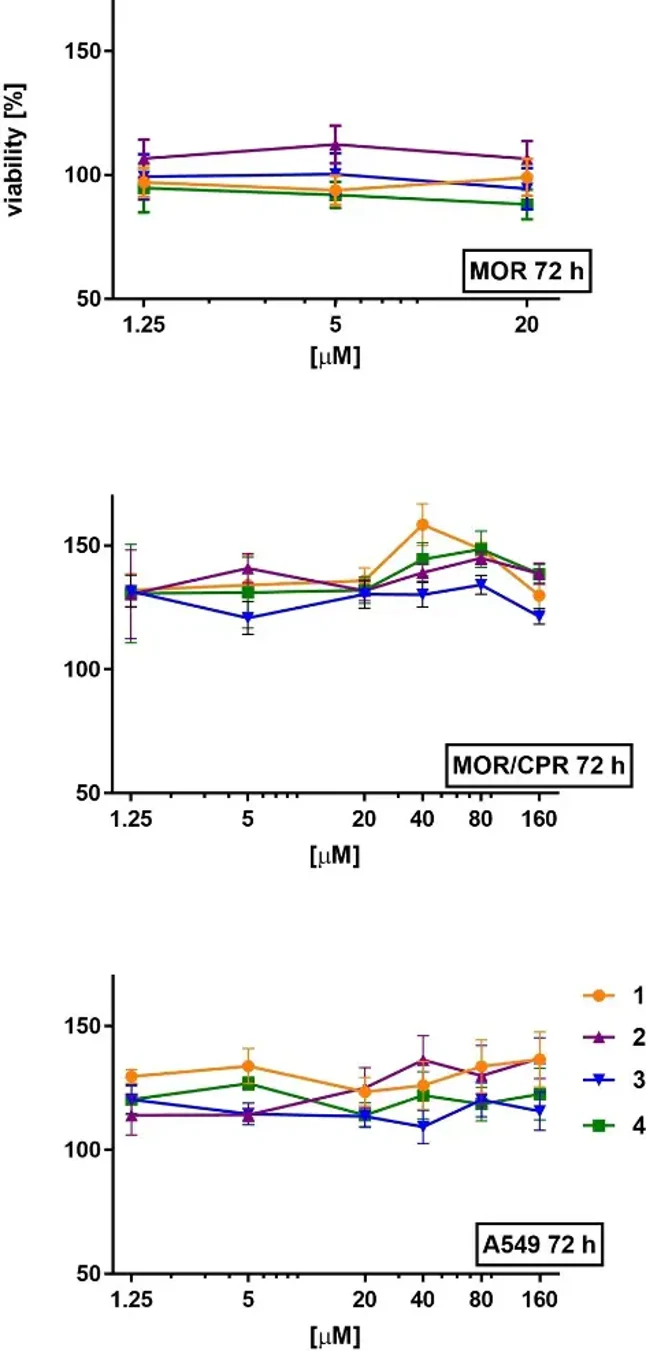
In Vitro Cytotoxicity of Dinuclear Complexes
Dinuclear complexes of the general formula [M(μ-X)(η5-Cp*)X]2 (M=Rh or Ir, X=Br or I) are important intermediates for preparing biologically and catalytically active half-sandwich complexes. Petrželová et al. optimized rapid microwave-assisted syntheses of the mentioned dinuclear synthons 1-4 and evaluates their antiproliferative activity in human cancer cell lines and catalytic activity in transfer hydrogenation.
As mentioned, complexes 1-4 can be used to synthesize biologically active Rh and Ir bromido and iodido complexes. To evaluate their biological activity, they tested complexes 1-4 on lung cancer cell lines (A549, MOR, and MOR/CPR; Fig. 1). The synthetic precursors (HCp*, RhCl3⋅xH2O, and IrCl3⋅xH2O) showed no cytotoxic effects on A549 cells after 72 h of incubation (data not shown). The antiproliferative effects of the bromido and iodido analogues 1-4 were not previously studied. They found that 1-4 had no significant cytotoxic effects on A549 and MOR/CPR cell lines up to 160 μM. A slight decrease in viability (84%) was observed in MOR cells treated with 20 μM of Ir iodido complex 4, but this is not considered cytotoxic (IC50 < 10 μM as per NIH criteria). Ir complex 4 showed higher antiproliferative activity than its Rh analogue (3), consistent with previous findings for Ir and Rh chlorido dimers. Cytotoxic effects were only detectable with long incubation times and high concentrations. Given their negligible cytotoxicity, compounds 1-4 can be used to synthesize biologically active half-sandwich cyclopentadienyl Rh(III) and Ir(III) compounds.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells