C8-D1A
Cat.No.: CSC-C9092W
Species: Mus musculus (Mouse)
Source: Brain
Morphology: neuronal
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
C8‑D1A is a spontaneously immortalized astrocyte cell line from the cerebellum of an 8‑day‑old C57BL/6 mouse. The cells are GFAP-positive and are part of the astrocytic cell lineage. C8‑D1A has three morphologically distinct GFAP-positive clones: Type I (small soma, with many short processes), Type II (small soma with a single very thin and long process), and Type III (larger, flat soma with no processes). Morphologically, the cells are adherent to the culture surface and display a neuronal‑like morphology with short neurites. They grow as a monolayer.
C8‑D1A astrocytes have been used extensively to model neuroinflammatory responses, glial scar formation, and astrocyte‑mediated neurotransmitter regulation. They are also susceptible to a number of mouse scrapie strains (22L, RML), and have been used in prion research. The line was recently used to characterize JAK2/STAT3 activation as part of the signaling events in methylmercury toxicity, and so the cell line can also be used for mechanistic toxicology and drug‑screening assays. Since the cells maintain many astrocytic properties (glutamate uptake, cytokine secretion, neuronal survival), the cells can be used for mechanistic studies and evaluation of potential therapeutics for neurodegenerative disease, CNS injury, and more.
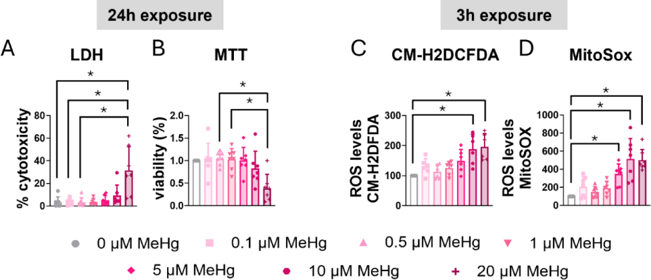
Exposure To MeHg Induces Mortality and Stress Oxidative in C8-D1A Astrocytic Cells
MeHg poisons astrocytes despite early Nrf2 activation. Using C8-D1A cells, Ahmed et al. blocked STAT3 with AG490/C188-9 and applied ROS scavengers NAC/Trolox to ask if STAT3 curbs acute toxicity and how ROS modulate its activation.
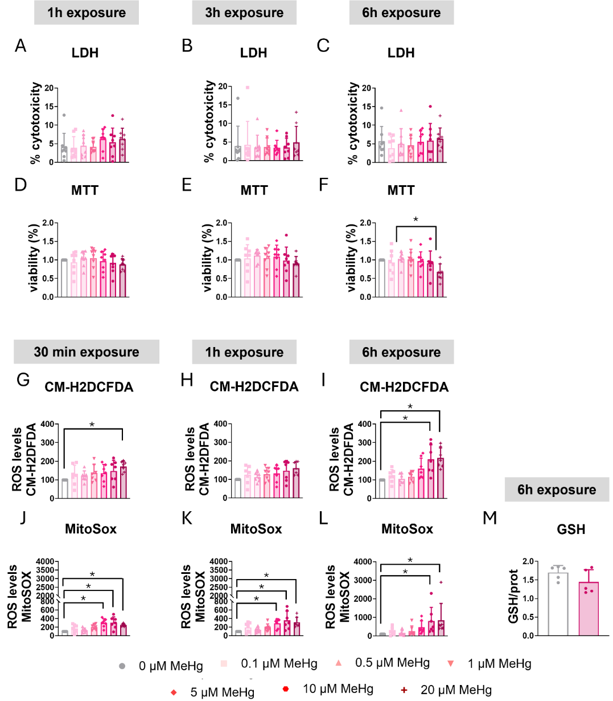
C8-D1A astrocytic cells were exposed to various MeHg concentrations, and cytotoxicity was evaluated with LDH assay. After 24 h, 20 µM MeHg significantly increased LDH release (H=19.926, p=0.003; post hoc p=0.006 vs non-exposed, Fig. 1A); shorter exposures or lower concentrations had no effect (Fig. 2A-C). MTT assay corroborated these findings: 24 h exposure to 20 µM MeHg significantly reduced cell viability in a concentration-dependent manner (H=18.351, p=0.005, Fig. 1B), whereas lower concentrations and shorter times did not (Fig. 2D-F).
MeHg is known to induce oxidative stress; therefore, ROS production was measured with CM-H2DCFDA. Twenty µM MeHg elevated ROS at 3 h (H=21.927, p=0.001; post hoc p=0.006, Fig. 1C) and also at 30 min (Fig. 2G) and 6 h (Fig. 2I). Ten µM MeHg significantly increased ROS at both 3 h and 6 h as well. Because MeHg disrupts mitochondrial function and increases mitochondrial superoxide, they used MitoSOX Red. Twenty µM MeHg significantly raised mitochondrial superoxide at 3 h (H=33.564, p<0.001, Fig. 1D), with similar results at 30 min, 1 h and 6 h (Fig. 2J-L); 5 µM was already effective at 30 min. To further assess oxidative stress, intracellular glutathione (GSH) was measured. Although MeHg is reported to deplete GSH, 10 µM MeHg did not significantly alter total GSH levels after 6 h (Fig. 2M).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells