A-253
Cat.No.: CSC-C9152W
Species: Homo sapiens (Human)
Source: Salivary Gland
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The A-253 human epithelial cell line is a long-established, widely used and characterized cell line originally derived from a salivary gland epidermoid carcinoma of submaxillary gland. The cell line is an established in vitro model for studies of salivary gland tumor biology and squamous cell carcinoma-like epithelial malignancies. A-253 cells exhibit a characteristic epithelial morphology, growing as an adherent monolayer of polygonal cells with distinct cell-cell contact. For these reasons, the A-253 cell line has also been used for studies into epithelial differentiation, adhesion and tumor progression.
At the genetic and phenotypic level, the A-253 cell line has been demonstrated to maintain many of the properties of malignant epithelial cells, such as deregulated proliferation and disrupted signaling pathways that are important in cancer cell growth and survival. The A-253 cell line has been broadly utilized in the study of oncogenic signaling, cell cycle and apoptosis regulation, and cellular response to therapeutics in the specific context of head and neck-related malignancy. The cell line has also been used for the investigation of drug sensitivity, radiation response and molecular mechanisms of chemoresistance.
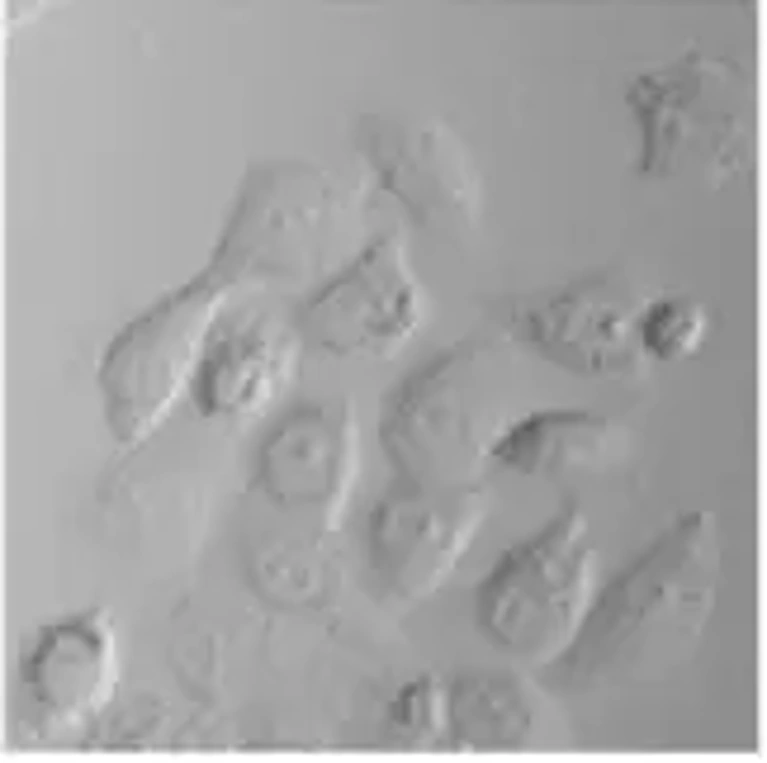
In vitro Cytotoxicity and Cell Viability Analysis of Ceb-Cu-p-NC
Head and neck squamous cell carcinomas (HNSCCs) require precise treatments. Cetuximab (Ceb) targets EGFR, and copper (Cu) compounds show promise in cancer therapy. AIFay et al. investigated Ceb-Cu-p-NC, a nanoengineered drug delivery system, designed for enhanced HNSCC treatment.
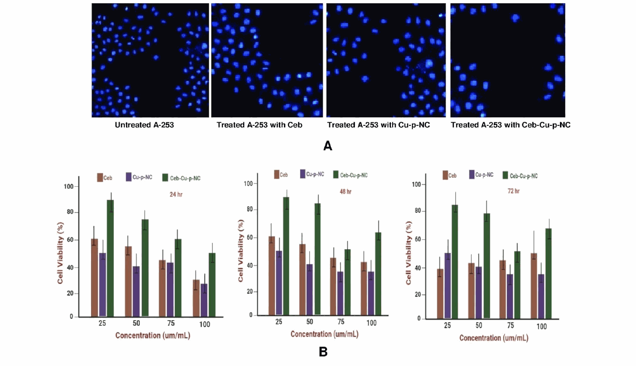
After treating A-253 cells with free Ceb, Cu-p-NC, and Ceb-Cu-p-NC, cell viability was assessed using DAPI staining and fluorescence microscopy. Figure 1A shows reduced fluorescence intensity in small hemispherical nuclei following treatment. Optimal doses of these formulations significantly increased apoptosis, with Ceb-Cu-p-NC showing a more pronounced effect than free Ceb. Specifically, 25 µg/mL of Ceb-Cu-p-NC reduced A-253 cell viability, indicating persistent DNA damage. The IC50 values for Ceb in Ceb-Cu-p-NC were 27.55 µg/mL, 41.35 µg/mL, and 51.47 µg/mL after 24, 48, and 72 hours, respectively. Figures 1A and 1B illustrate a time- and dose-dependent anticancer effect against the A-253 cell line. Ceb-Cu-p-NC's controlled release from 24 to 72 hours resulted in higher cytotoxicity than free Ceb, achieving approximately 81.25% reduced cell viability at different concentrations. Therefore, the optimal concentration of Ceb-Cu-p-NC is 25 µg/mL to achieve nearly 80% of reduced cell viability (Fig. 1B). Copper compounds have a potential anticancer effect on their own or in combination with other drugs by altering the DNA regulation processes, cell cycle checkpoints, and apoptotic pathways. It can be concluded that Cu-based polymeric modifications are potent in targeted anticancer drug delivery with different release patterns. Folate-decorated nanostructured lipid carriers for the co-delivery of cisplatin and paclitaxel exhibit excellent anticancer effects in head and neck cancer. The obtained results will make this carrier a potential future work for a dual-drug delivery system.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells