Rabbit Osteoblasts
Cat.No.: CSC-C5212S
Species: Rabbit
Source: Bone
Cell Type: Osteoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rabbit osteoblasts from Creative Bioarray are isolated from the rabbit bone tissue. The method we use to isolate rabbit osteoblasts was developed based on a combination of established and our proprietary methods. The rabbit osteoblasts are characterized by alkaline phosphatase (ALP) staining.. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
The rabbit osteoblasts cell line develops from bone tissue collected from rabbit skulls as well as their long bones such as femur and tibia and spine. These cells are located mainly in the periosteum and endosteum as well as within the bone matrix. The rabbit osteoblasts demonstrate standard osteoblast shape as single-nucleus cells that generate both alkaline phosphatase (ALP) and osteocalcin (OC) markers. These cells adhere to surfaces to form mineralized structures while developing the bone matrix by secreting type I collagen and osteopontin. They also play vital roles in regulating bone metabolism pathways including Wnt/β-catenin signaling and both BMP and RANKL/OPG pathways.
Rabbit osteoblasts serve as a valuable research tool in multiple scientific fields:
Bone Biology Research: Rabbit Osteoblasts are widely used to study the molecular mechanisms of bone formation, metabolism, and remodeling.
Bone-Related Disease Models: These cells can be used to investigate the pathogenesis of bone-related diseases such as osteoporosis, fracture healing, and osteoarthritis.
Drug Screening: Rabbit Osteoblasts are used to screen and evaluate drugs that promote bone formation or inhibit bone resorption, such as bisphosphonates, parathyroid hormone (PTH), and vitamin D analogs.
Puerarin Attenuated Dex-Induced Inhibition on the Osteoblastic Differentiation of Rabbit Osteoblasts Partially by Increasing miR-34a Expression
Steroid-induced necrosis of the femoral head (SONFH) develops through progressive bone cell death triggered by extended or high-dose glucocorticoid treatment. Glucocorticoids trigger vasoconstriction and fatty emboli production which leads to osteoblast apoptosis and blocks bone repair and remodeling. Osteoblasts function as essential elements in bone growth and remodeling with their dysfunction resulting in SONFH development. The isoflavonoid phytoestrogen puerarin has demonstrated the ability to enhance bone formation. Jiang's team identified how puerarin stimulates osteoblastic differentiation and prevents SONFH by upregulating miR-34a.
Researchers established in vitro SONFH models to study whether puerarin's protective effect on SONFH rabbits depends on enhanced osteoblastic differentiation and elevated miR-34a by treating cells with Dex (1 μM) alone or with puerarin (0.1 μM) and miR-34a mimic/mimic control. Dex reduced miR-34a expression and osteoblast viability but puerarin lessened this suppressive effect (Fig. 1A and B). The introduction of miR-34a mimic amplified the beneficial outcomes of puerarin treatment as demonstrated in Figure 1A and B. 1A and B). Dex decreased red mineralized nodules and reduced levels of RUNX2, COL1A1, and ALP proteins but puerarin attenuated these effects (Fig. 1C–E). The administration of miR-34a mimic transfection amplified the protective benefits of puerarin (Fig. 1C–E). The results demonstrate that puerarin fights against SONFH through osteoblastic differentiation by increasing miR-34a levels.
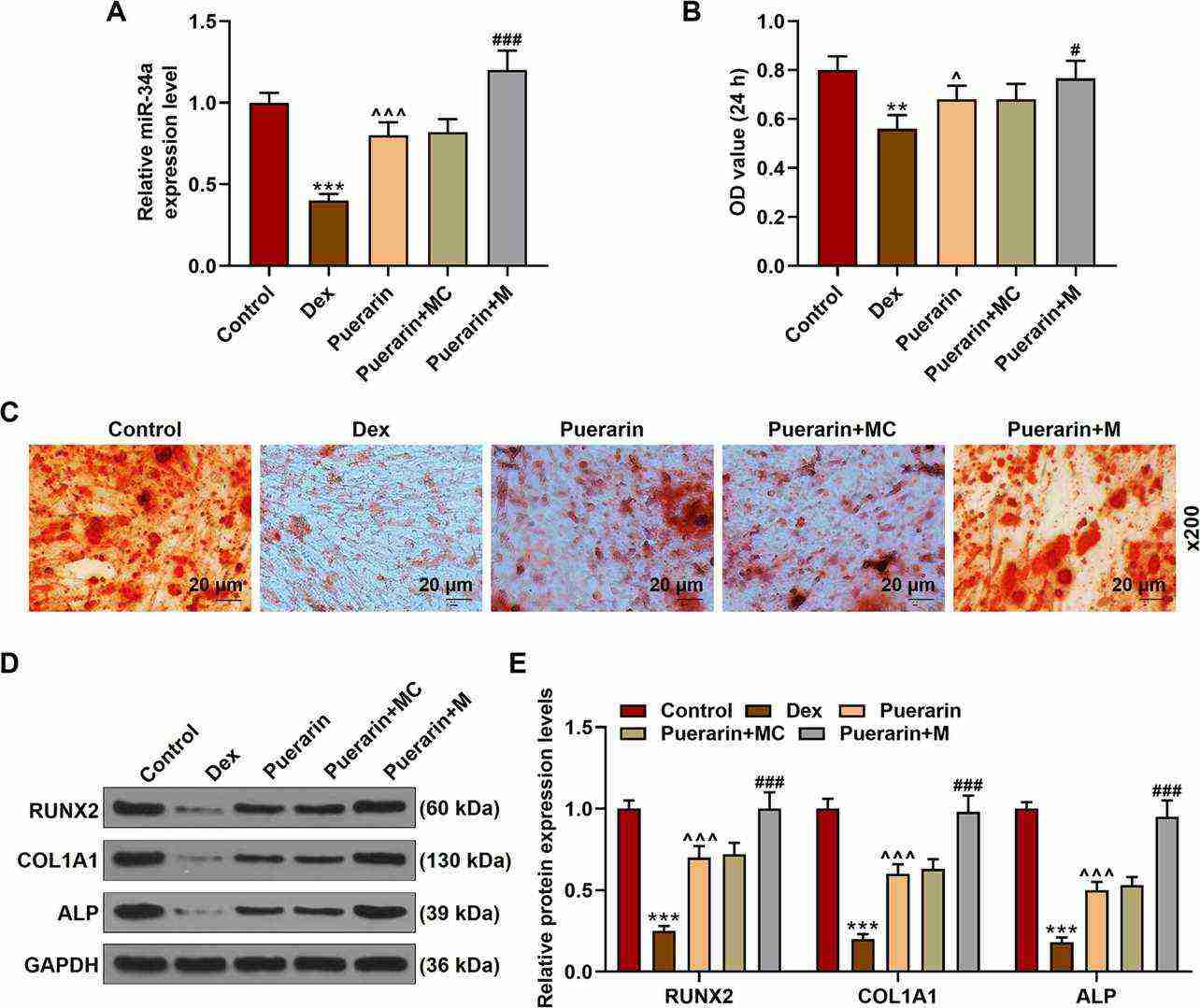 Fig. 1. Puerarin attenuated Dex-induced inhibition on the osteoblastic differentiation of rabbit osteoblasts partially by increasing miR-34a expression (Jiang X, Chen W, et al., 2021).
Fig. 1. Puerarin attenuated Dex-induced inhibition on the osteoblastic differentiation of rabbit osteoblasts partially by increasing miR-34a expression (Jiang X, Chen W, et al., 2021).
Effect of the SF/HA Hybrid Coating on Osteoblast Adhesion and Morphology under Diabetic Conditions
Titanium implants are widely used for bone repair due to their excellent properties, but their performance is compromised in diabetic patients due to impaired osseointegration. Previous studies have shown that ROS overproduction inhibits the PI3K/Akt signaling pathway, which is crucial for osteoblast function. Silk fibroin (SF) and hydroxyapatite (HA) have been used to improve bone regeneration. Ma's team explored whether a silk fibroin/hydroxyapatite (SF/HA) coating on titanium implants can improve osseointegration in diabetic conditions by reactivating the PI3K/Akt signaling pathway.
To analyze osteoblast adhesion, vinculin, rhodamine phalloidin, and DAPI triple staining were performed on osteoblasts from all groups on day 7 (Fig. 2A). Vinculin immunostaining showed high fluorescence intensity (11.8 ± 1.2 au) in osteoblasts on TI with normal serum (NS), while diabetic serum (DS) significantly decreased it to 3.5 ± 0.5 au. The silk fibroin/hydroxyapatite (SF/HA) - coated titanium implant (SHT) substrate restored vinculin intensity to 7.8 ± 1.8 au (Fig. 2B). Cytoskeleton spreading was assessed by the cellular area/nuclear (CN) ratio. Osteoblasts on TI with NS had a large CN ratio (15.0 ± 3.1), while DS reduced it to 4.0 ± 1.1. SHT improved the CN ratio to 9.6 ± 2.2 (Fig. 2C). Scanning electron microscopy (SEM) further visualized osteoblast morphology on day 7 (Fig. 3). Osteoblasts on TI with NS had a widespread, flat shape, while DS caused sparse atrophy. SHT improved osteoblast morphology with elongated shapes and filopodia anchoring to the substrate (white arrow), indicating better adhesion.
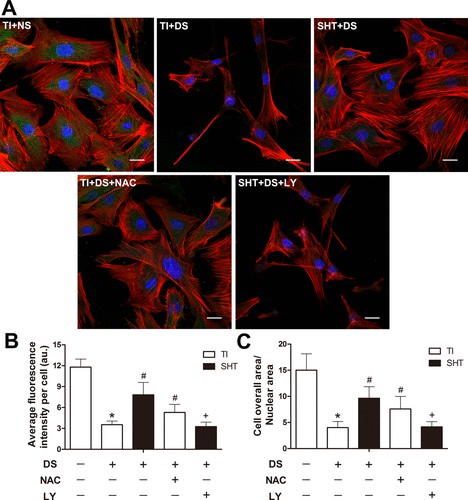 Fig. 2. Assessment of the osteoblast actin cytoskeleton in each group on the seventh day of incubation by fluorescent labeling by vinculin (green), rhodamine-phalloidin (red), and DAPI (blue) (Ma X, Cui D, et al., 2022).
Fig. 2. Assessment of the osteoblast actin cytoskeleton in each group on the seventh day of incubation by fluorescent labeling by vinculin (green), rhodamine-phalloidin (red), and DAPI (blue) (Ma X, Cui D, et al., 2022).
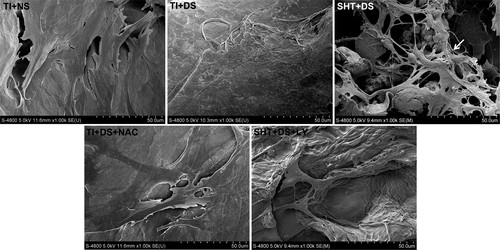 Fig. 3. Observation of osteoblast morphology by SEM in each group on the seventh day of incubation (Ma X, Cui D, et al., 2022).
Fig. 3. Observation of osteoblast morphology by SEM in each group on the seventh day of incubation (Ma X, Cui D, et al., 2022).
Ask a Question
Write your own review