Rabbit Lens Epithelial Cells
Cat.No.: CSC-C5172S
Species: Rabbit
Source: Lens; Eye
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rabbit lens epithelial cells from Creative Bioarray are isolated from the rabbit eye tissue. The method we use to isolate rabbit lens epithelial cells was developed based on a combination of established and our proprietary methods. The rabbit lens epithelial cells are characterized by immunofluorescence with antibodies specific to cytokeratin-18 (CK-18). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rabbit Lens Epithelial Cells (RLECs) are commonly used for research related to the eye. They are polygonal or spindle-shaped cells that make up a monolayer located between the lens capsule and the lens substance. Their roles include antioxidant defense (express high levels of SOD/GSH-Px) and autophagy-mediated protein degradation to maintain lens transparency. RLECs are often utilized to study the pathogenesis of cataracts (e.g., oxidative stress models such as H₂O₂/UVB-induced damage) and for drug discovery/screening for potential therapeutics like flavonoids. They are also used to investigate epithelial-mesenchymal transition (EMT) in posterior capsule opacification (PCO). RLECs are typically cultured in DMEM/F12 supplemented with EGF. While they have a limited lifespan (3–5 passages), immortalized RLEC lines (e.g., SRA01/04) are available. The advantages of using RLECs include high human relevance to lens physiology and relatively easy isolation from large rabbit lenses. Disadvantages include the short lifespan of primary cells and potential differences in certain pathways between species. RLECs are crucial for drug toxicity screening, gene editing studies (e.g., CRISPR knockout of antioxidant genes), and understanding the molecular mechanisms of lens homeostasis.
Molecular Characteristics of Lens Fibrosis and TGFβ2-Stimulated LECs
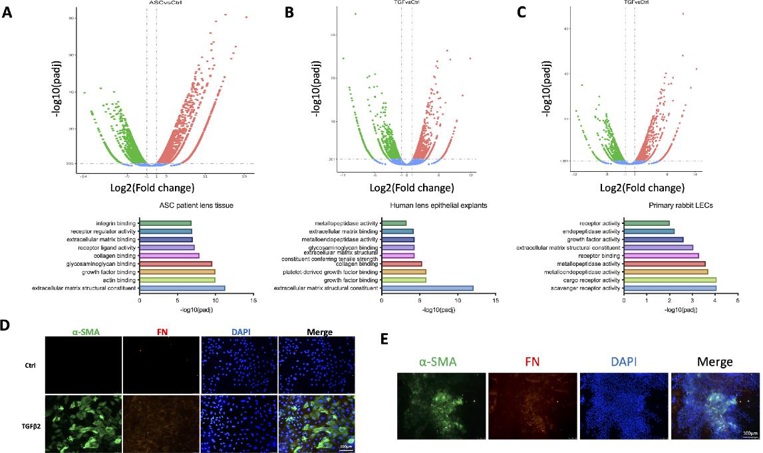
Pathological epithelial–mesenchymal transition (EMT) of lens epithelial cells (LECs) is the underlying pathogenesis of lens fibrosis such as fibrotic posterior capsular opacification and anterior subcapsular cataract (ASC). Here, Wang’s team investigated the potential roles of endoplasmic reticulum (ER) stress in the development of lens fibrosis.
They performed RNA-seq of lens capsules from normal individuals and ASC patients to explore the molecular characteristics of lens fibrosis. EMT-related genes were highly upregulated in ASC samples, and structural components of the extracellular matrix (ECM) and genes encoding for growth factor binding were activated (Fig. 1A). As TGFβ2 is the most important initiator of lens fibrosis, they performed RNA-seq of human lens explants (Fig. 1B) and rabbit LECs (Fig. 1C) following treatment with TGFβ2. Significantly changed pathways were highly similar to those seen in patient samples. TGFβ2 stimulation of LECs caused upregulation of FN and α-SMA and a switch to mesenchymal morphology (Fig. 1D). They have also developed a mouse model of injury-induced capsular fibrosis, which mimics post-injury or post-cataract surgery lens fibrosis, and showed expression of α-SMA and fibronectin in lens epithelial cells (Fig. 1E). These mouse models and TGFβ2-stimulated LECs were then used as platforms to study the mechanisms and therapeutic targets of lens fibrosis.
Effects of H2O2 and BMFs on the Synergistic Consequence of Cells
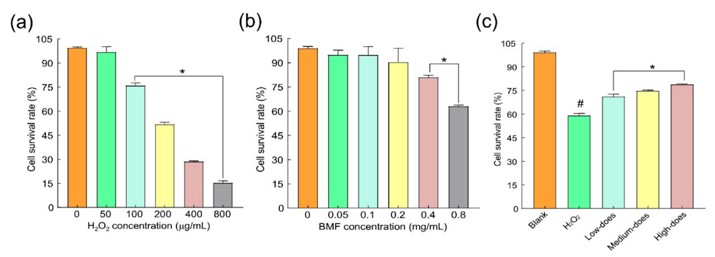
Cataracts, the leading cause of global blindness, lack effective treatments. Oxidative stress and impaired autophagy in lens epithelial cells are key factors in cataract development. Buddleja officinalis Maxim flavonoids (BMF), known for their antioxidant and anti-inflammatory properties, may offer therapeutic potential. Wei’s team optimized BMF extraction, identify its active compounds (luteolin, apigenin, acacetin), and evaluate BMF’s protective effects against H2O2-induced oxidative damage in rabbit lens cells by modulating autophagy and antioxidant responses.
Guan et al. reported that low-concentration H2O2 (1–10 nmol/L) promotes human lens epithelial cell proliferation, whereas high concentrations (>100 μmol/L) induce cytotoxicity. Based on preliminary data, they tested 0–800 μmol/L H2O2 and identified 200 μmol/L as the near-IC₅₀ dose (Fig. 2a). Based on the literature and pre-experimental results, the concentration gradient of BMFs was set from 0.05 mg/mL to 0.8 mg/mL, and the CCK-8 method was used to detect the cytotoxic effect of different concentrations of BMF on normal rabbit lens epithelial cells. BMF ≤ 0.2 mg/mL showed no cytotoxicity, while 0.4 mg/mL reduced cell viability. Thus, 0.05, 0.1, and 0.2 mg/mL were selected as low, medium, and high doses (Fig. 2b). H2O2 (200 μmol/L) significantly decreased cell survival vs. controls (p < 0.01), but all BMF doses improved viability (p < 0.01). Specifically, 0.05–0.2 mg/mL BMFs elevated survival rates vs. H2O2-injured cells (p < 0.05), confirming their protective effect (Fig. 2c).
Ask a Question
Write your own review
- You May Also Need
