HeLa.S3
Cat.No.: CSC-C6589J
Species: Homo sapiens (Human)
Source: Uterus; Cervix
Morphology: Other
Culture Properties: Suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Shipping: Dry Ice, Frozen
HeLa S3 is one of the most commonly used sub‑clones of the HeLa cervical‑cancer cell line, which was first established in 1955 from the cervical carcinoma of a ~30‑year‑old African‑American woman. The cells contain integrated human papillomavirus type 18 (HPV‑18) DNA, and a highly aneuploid karyotype (modal chromosome number ≈68). HeLa S3 retains the typical hallmarks of the parent HeLa cells, including rapid proliferation, loss‑of‑function of p53, and over‑expression of c‑Myc. HeLa S3 is grown adherently in MEM/EMEM or Ham's F‑12K media with 10 % fetal bovine serum, 1 % penicillin‑strept omycin, at 37 °C with 5 % CO₂.
Morphologically, HeLa S3 are polygonal to round in shape, resembling epithelial cells, and have large, densely stained nuclei. HeLa S3 is commonly used for transient or stable transfection studies due to its high transfection efficiency, as well as a model for studying a wide range of viruses including poliovirus, adenovirus and HPV. The HeLa S3 cell line is also commonly used for drug‑screening assays, apoptosis and cell‑cycle studies.
Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Cervical Cancer Cells Hela S3
Aphrocallistes vastus lectin (AVL) is a C-type marine lectin produced by sponges. The previous study demonstrated that genes encoding AVL enhanced the cytotoxic effect of oncolytic vaccinia virus (oncoVV) in a variety of cancer cells. In this study, the inhibitory effect of oncoVV-AVL on Hela S3 cervical cancer cells, a cell line with spheroidizing ability, was explored.
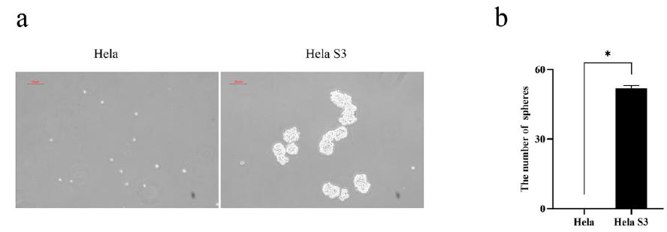
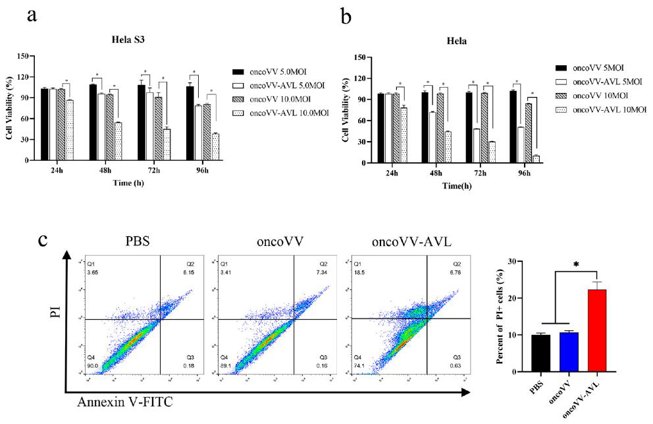
To compare the oncogenic properties of Hela and Hela S3 cells, a tumor sphere formation assay was conducted. Hela S3 cells continuously formed spheres through multiple passages, while Hela cells did not (Fig. 1a, b). Sphere-forming activity is indicative of cellular stemness. Given the stemness of Hela S3 cells in vitro, they were selected for further study. An MTT assay was performed to assess the cytotoxic effects of oncoVV-AVL on Hela S3 and Hela cells. Cells were infected with oncoVV or oncoVV-AVL, and viability was measured at 24, 48, 72, and 96 h (Fig. 2a, b). OncoVV-AVL exhibited significantly higher cytotoxicity in a dose- and time-dependent manner compared to oncoVV, indicating that AVL enhances the cytotoxicity of the oncolytic vaccinia virus. Flow cytometry was used to confirm the cytotoxic effects of oncoVV-AVL on Hela S3 cells by detecting apoptotic/dead cells infected with oncoVV or oncoVV-AVL (Fig. 2c). OncoVV-AVL induced higher cytotoxicity than oncoVV and PBS controls. However, there was no significant difference in the percentage of Annexin V-FITC positive apoptotic cells among the groups, suggesting that AVL promotes nonapoptotic cell death induced by oncolytic vaccinia viruses.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells