
Human Skeletal Muscle Satellite Cells (HSkMSC)
Cat.No.: CSC-7712W
Species: Human
Source: Skeletal Muscle
Cell Type: Satellite Cell; Myosatellite Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Skeletal Muscle Satellite Cells (HSkMSC) are primary myogenic progenitor cells, which were originally isolated from human thoracic or scapular skeletal muscle. These cells reside between the basal lamina and the sarcolemma of mature myofibers and make up about 1 % of total nuclei inside a muscle fiber. HSkMSCs are inactive under basal conditions, but become activated by muscle injury or mechanical overload. Morphologically, HSkMSCs are small, mononucleated, spindle‑shaped cells (10-15 µm) that express characteristic markers such as PAX7, CD56, CD29, and lack hematopoietic markers CD45 and CD34. Under in vitro conditions, they will proliferate well in the presence of a specialized satellite‑cell medium, reaching a confluence of 60-80 % within 48 h. HSkMSCs are expandable in culture for up to ~10-12 passages, while still retaining the ability to differentiate into myofibers. Upon differentiation, they fuse into multinucleated myotubes that express MyHC. Additionally, HSkMSCs have been shown to have adipogenic and osteogenic potential upon specific induction, suggesting a multipotent mesenchymal phenotype. Functionally, HSkMSCs are the only source of new myofibers in adult muscle regeneration. And they have also been shown to interact with fibro‑adipogenic progenitors to mediate fibrosis and fatty infiltration of muscle.
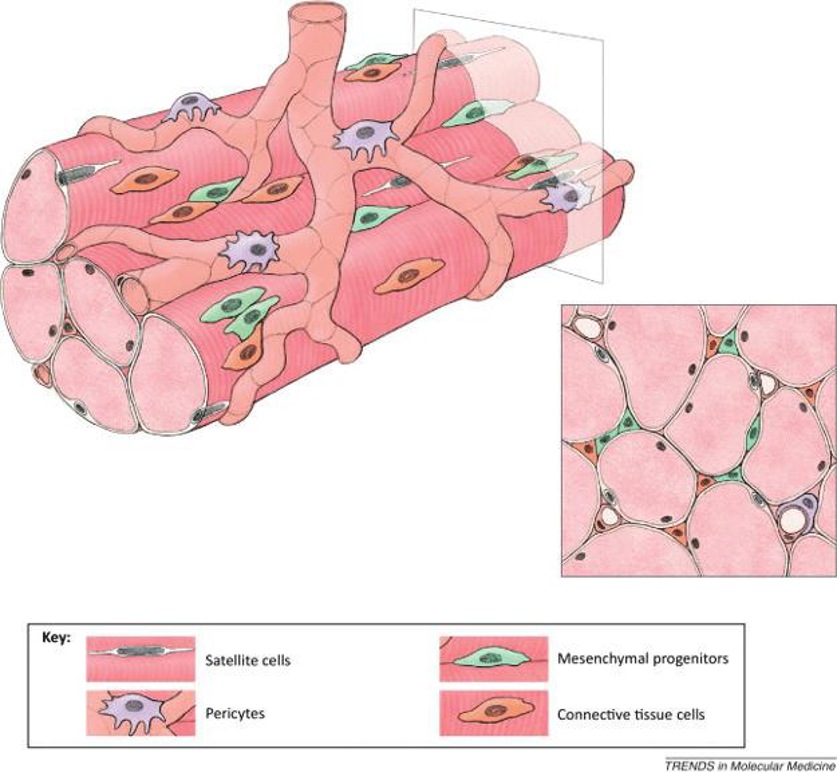
Cultured Human Skeletal Muscle Satellite Cells Exhibit Characteristics of Mesenchymal Stem Cells
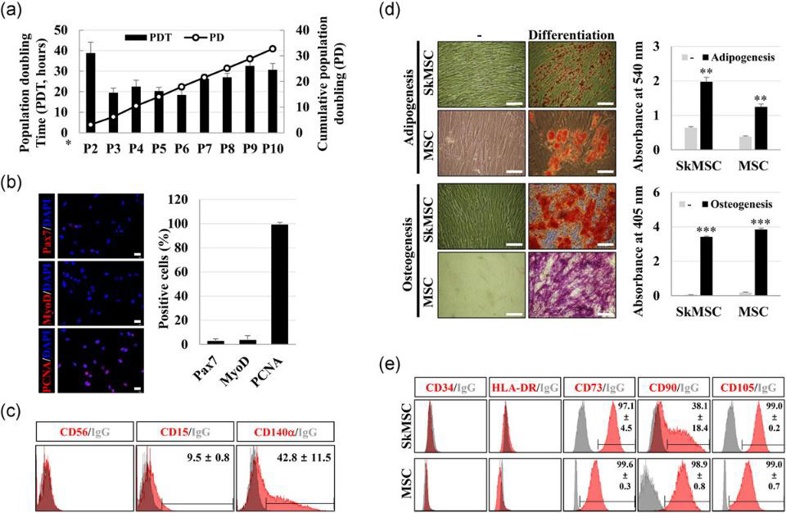
Skeletal muscle satellite cells (SkMSCs) play crucial roles in muscle fiber maintenance, repair, and remodeling; however, it remains unknown if these properties are preserved in cultured SkMSCs. In this study, Kim et al. investigated the characteristics of cultured SkMSCs and their ability to regulate the activity of M1 macrophages.
To verify if the cultured SkMSCs retained satellite cell characteristics, the expression of myogenic markers CD56, Pax7, and MyoD, as well as proliferation potential, were assessed. In serial subcultures up to P10, except for P2, the population doubling time remained around 20-30 h, with a total of 32.7 population doublings (Fig. 1a). At P5, SkMSCs showed low expression of Pax7 and MyoD (Fig. 1b) and did not express CD56. Instead, they expressed CD15 and CD140, markers of FAPs, in 9.5 ± 0.8% and 42.8 ± 11.5% of cells, respectively (Fig. 1c). This indicates that SkMSCs lost satellite cell characteristics and became fibroblast-like cells distinct from FAPs. Next, the differentiation capacity of SkMSCs and MSCs into adipocytes and osteoblasts was compared. SkMSCs differentiated more efficiently than MSCs (Fig. 1d). At P5, SkMSCs expressed CD73 (97.1 ± 4.5%), CD90 (38.1 ± 18.4%), and CD105 (99.0 ± 0.2%), but not CD34 or HLA-DR (Fig. 1e). These findings suggest that SkMSCs have similar differentiation and surface marker expression to MSCs.
Quercetin Induces a Slow Myofiber Phenotype in Engineered Human Skeletal Muscle Tissues
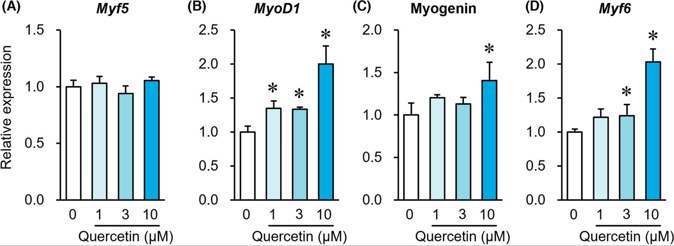
Slow and fast myofibers coexist in skeletal muscle, and slow myofibers are more adept at aerobic metabolism and endurance. Quercetin is a polyphenol compound that has been demonstrated to induce slow myofibers in rodent skeletal muscle. However, whether quercetin also elicits slow myofibers in human myofibers is not known. Here, Nagai et al. evaluated the effects of quercetin on the induction of slow myofibers in human skeletal muscle satellite cells.
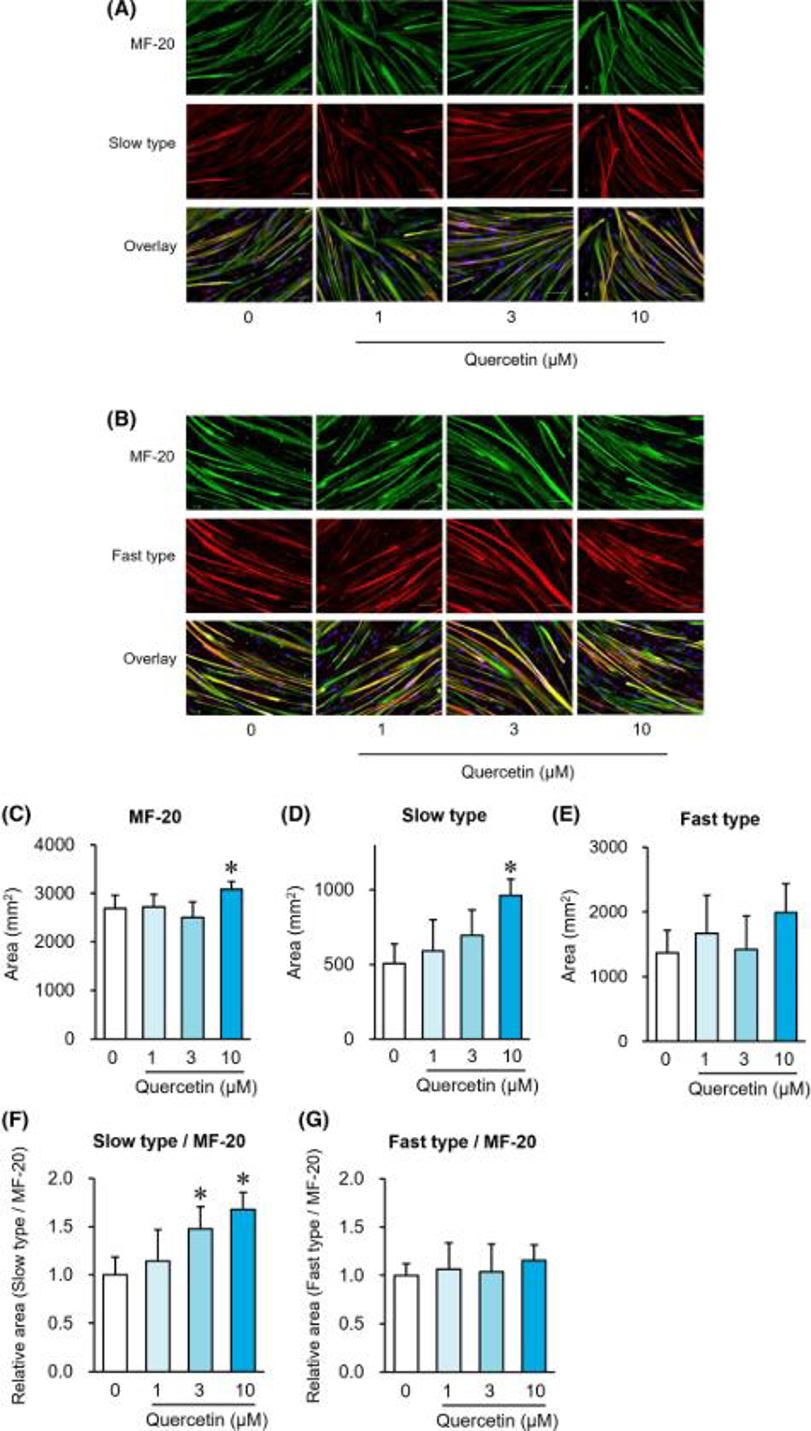
Muscle regulatory factors (MRFs) are involved in muscle differentiation and are used as markers for myofiber differentiation. Figure 2 depicts how quercetin alters the expression patterns of MRF genes within human muscle satellite cells by the eighth day. Quercetin had no effect on Myf5 gene expression (Fig. 2A), but it increased MyoD1 gene expression in a dose-dependent manner at 1, 3, and 10 μM (Fig. 2B). The expression of Myogenin (Fig. 2C) and Myf6 (Fig. 2D) genes was also significantly increased in a dose-dependent manner by quercetin at 10 μM and 3 and 10 μM, respectively. On Day 8, immunostaining of MF-20 (total MyHC) and slow or fast myofibers was performed (Fig. 3A, B). Quercetin significantly increased the area of MF-20-positive cells (Fig. 3C) and slow myofiber-positive cells (Fig. 3D), but not fast myofiber-positive cells (Fig. 3E). The area ratio of slow myofiber-positive cells to MF-20-positive cells rose noticeably following quercetin administration at 3 and 10 μM (Fig. 3F) and yet the fast myofiber-positive cells to MF-20-positive cells area ratio remained unaffected (Fig. 3G).
Ask a Question
Write your own review
