Human Adult Mesothelial Cells
Cat.No.: CSC-7683W
Species: Human
Source: Peritoneal Cavity
Cell Type: Mesothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mesothelial cells (MCs) form the superficial anatomic layer of serosal membranes, including pleura, pericardium, peritoneum, and the tunica of the reproductive organs. They produce a protective, non-adhesive barrier against physical and biochemical damages. MCs express multiple phenotypic markers, including vimentin and cytokeratins. They play key roles in fluid transport and inflammatory responses, as evidenced by the regulation of biochemical markers such as transporters, adhesion molecules, cytokines, growth factors, reactive oxygen species and their scavengers. MCs synthesize extracellular matrix related molecules, and the surface of MC microvilli secretes a highly hydrophilic protective barrier, "glycocalyx", consisting mainly of glycosaminoglycans. MCs maintain a balance between procoagulant and fibrinolytic activation by producing a range of regulators, enabling the synthesis of fibrin and therefore form adhesions. The synthesis and recognition of hyaluronan and sialic acids might be a new insight to explain immunoactive and immunoregulatory properties of mesothelial cells. Epithelial to mesenchymal transition of MCs may involve serosal repair and remodeling. MCs might also play a role in the development and remodeling of visceral adipose tissue. Taken together, MCs play crucial roles in health and disease in serosal cavities of the body. Researchers continue to investigate mesothelial cells, developing methods to regulate these processes with the goal of providing more effective treatments for disease.
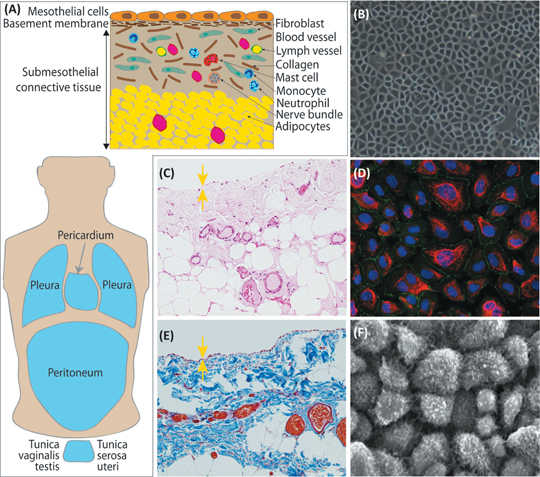 Fig. 1. The morphological characteristics of mesothelial cells (Kawanishi K. 2016).
Fig. 1. The morphological characteristics of mesothelial cells (Kawanishi K. 2016).
Creative Bioarray isolate peritoneal mesothelial cells from omental tissue biopsies with minimal propagation. Mesothelial cells are available either cryopreserved or in culture. Ready to use medium is also available for use with our mesothelial cells.
Autophagy Promotes TGF-β1-Induced Epithelial-To-Mesenchymal Transition in Human Peritoneal Mesothelial Cells
Epithelial-to-mesenchymal transition (EMT) is one of the main causes of peritoneal fibrosis. However, the pathophysiological mechanisms of EMT, specifically its relationship with autophagy, are still unknown. This study aimed to evaluate the role of autophagy in transforming growth factor-beta 1 (TGF-β1)-induced EMT in human peritoneal mesothelial cells (HPMCs).
Primary cultured HPMCs were treated with TGF-β1 (2 and 5 ng/mL) and changes in autophagy markers and the relationship between autophagy and EMT were evaluated. TGF-β1 increased the generation of NADPH oxidase 4 (NOX4) and reactive oxygen species (ROS) in HPMCs, resulting in mitochondrial damage. Treatment with GKT137831 (20 μM), a NOX1/4 inhibitor, reduced ROS in the mitochondria of HPMC cells and reduced TGF-β1-induced mitochondrial damage. Additionally, the indirect inhibition of autophagy by GKT137831 (20 μM) downregulated TGF-β1-induced EMT, whereas direct inhibition of autophagy using 3-methyladenine (3-MA) (2 mM) or autophagy-related gene 5 (ATG5) gene silencing decreased the TGF-β1-induced EMT in HPMCs. These results suggest that autophagy could serve as a therapeutic target for the prevention of peritoneal fibrosis in patients undergoing peritoneal dialysis.
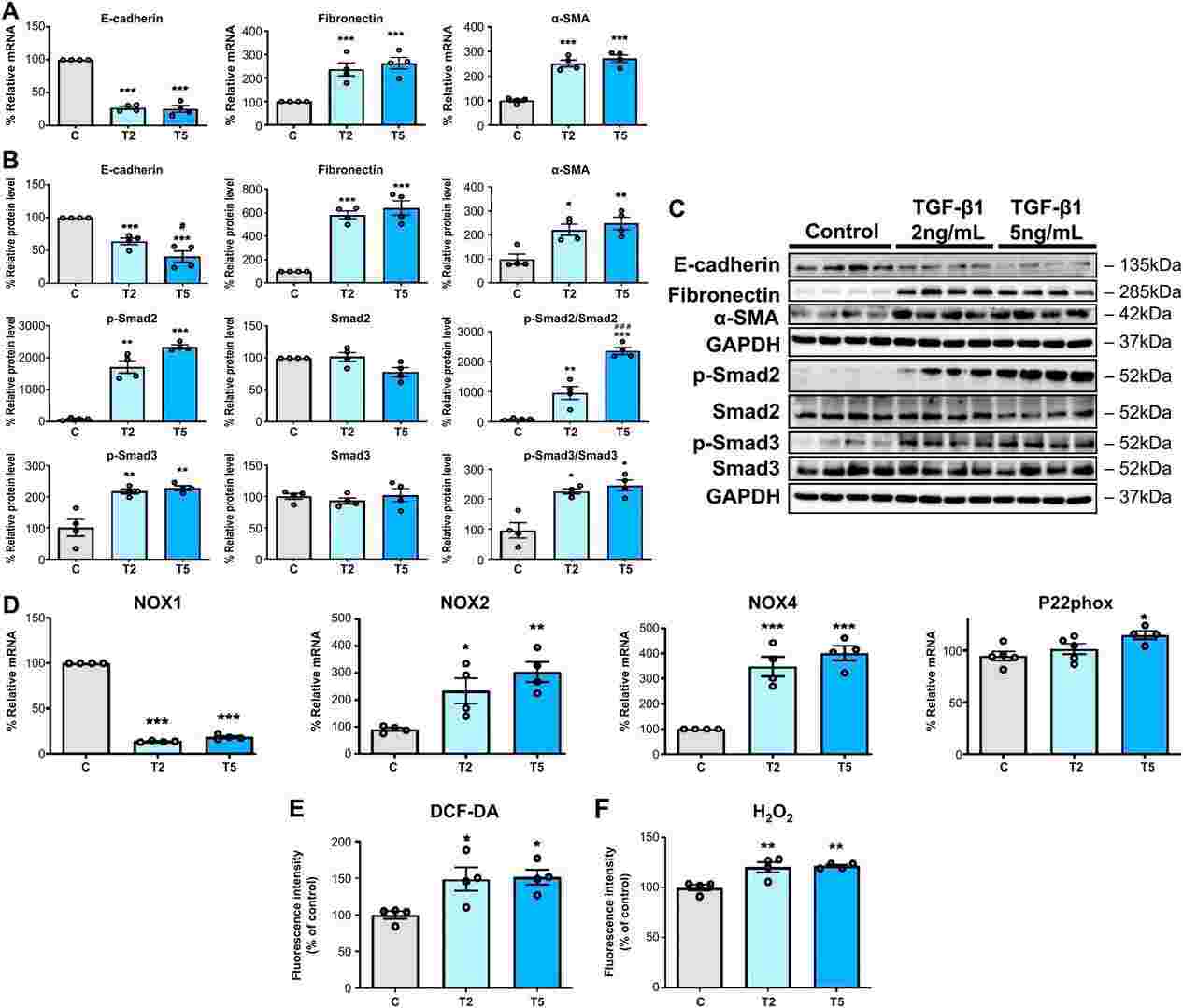 Fig. 1. TGF-β1 induces EMT and ROS generation in HPMCs (Oh, Se-Hyun, et al. 2024).
Fig. 1. TGF-β1 induces EMT and ROS generation in HPMCs (Oh, Se-Hyun, et al. 2024).
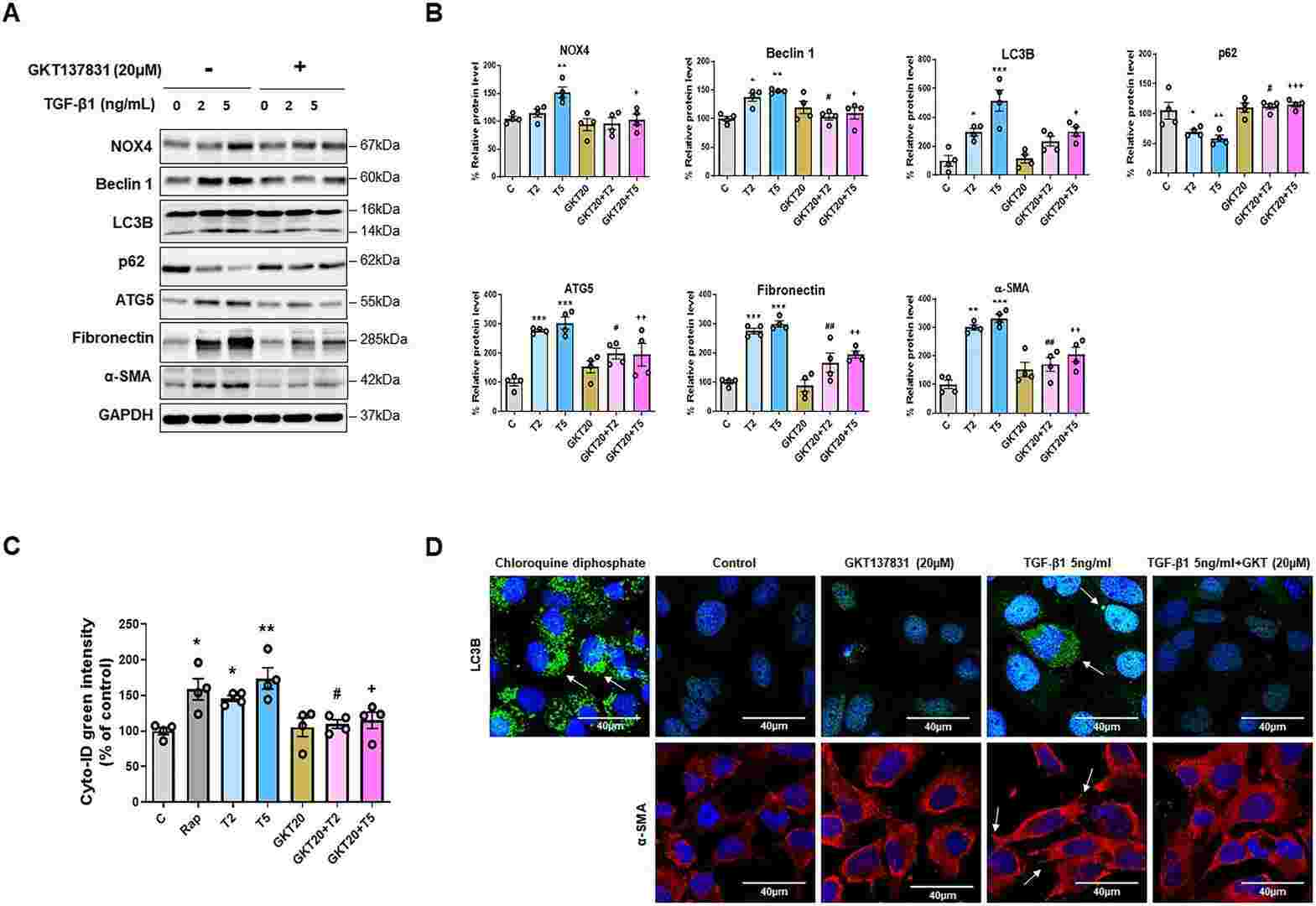 Fig. 2. NOX4 inhibition downregulates TGF-β1-induced autophagy and EMT in HPMCs (Oh, Se-Hyun, et al. 2024).
Fig. 2. NOX4 inhibition downregulates TGF-β1-induced autophagy and EMT in HPMCs (Oh, Se-Hyun, et al. 2024).
Human Omental Mesothelial Cells Exhibit a Corneal Endothelial-Like Cell Phenotype
To evaluate whether cultured human omental mesothelial cells (OMC) exhibit phenotypic and functional similarities to human corneal endothelial cells (CEC) and whether they can adhere to the corneal stroma and form a biomimetic corneal endothelium when grown on a human anterior lens capsule (HALC).
Human OMC were isolated from the greater omentum. Human B4G12 CEC were used as a reference for native and functional CEC phenotype whereas human mesenchymal stromal cells (MSC) served as a phenotypically distinct control group. OMC, CEC and MSC were compared using flow cytometry, immunofluorescence and qRT-PCR for lineage-specific markers, as well as through a transepithelial permeability assay. The adhesion capacity of OMC was also evaluated on both mouse corneal stroma and a decellularized HALC.
The results demonstrate that cultured human OMC exhibit phenotypic and functional similarities to CEC. The ability of OMC to adhere to the corneal stroma and to generate a biomimetic corneal endothelium when combined with HALC highlights their potential use in corneal endothelial regeneration.
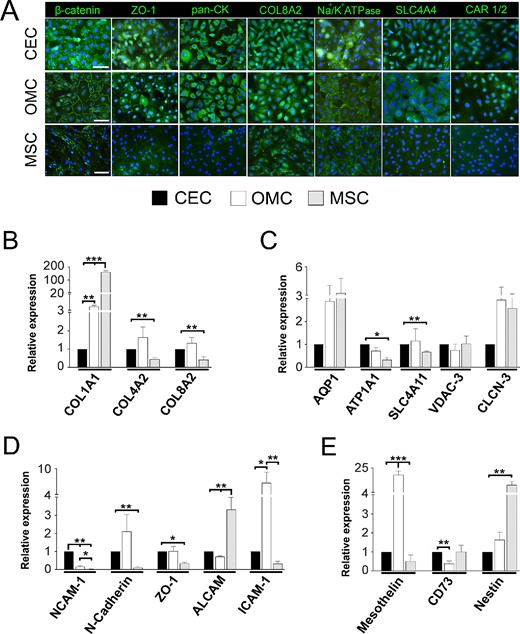 Fig. 3. OMC and CEC display high similarities in their immunoexpression patterns and gene expression levels of simple epithelium and corneal endothelium markers (Alió, Jorge L., et al. 2025).
Fig. 3. OMC and CEC display high similarities in their immunoexpression patterns and gene expression levels of simple epithelium and corneal endothelium markers (Alió, Jorge L., et al. 2025).
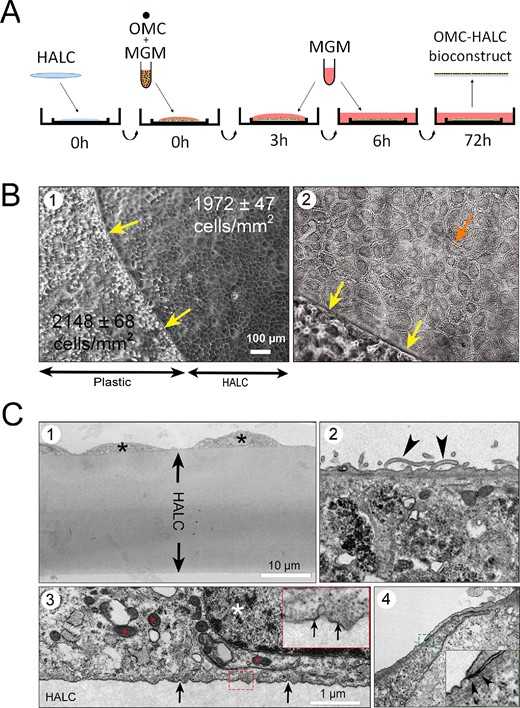 Fig. 4. HALC seeded with OMC creates a corneal endothelium biomimetic (Alió, Jorge L., et al. 2025).
Fig. 4. HALC seeded with OMC creates a corneal endothelium biomimetic (Alió, Jorge L., et al. 2025).
Ask a Question
Write your own review