C57BL/6 Mouse Bone Marrow Mesenchymal Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Bone Marrow Mesenchymal Stem Cells are negative for bacteria, yeast, fungi, and mycoplasma.Cells can be expanded on a multiwell culture platetandard biochemical procedures performed with cell ready for experiments under the cell culture conditions specified by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.
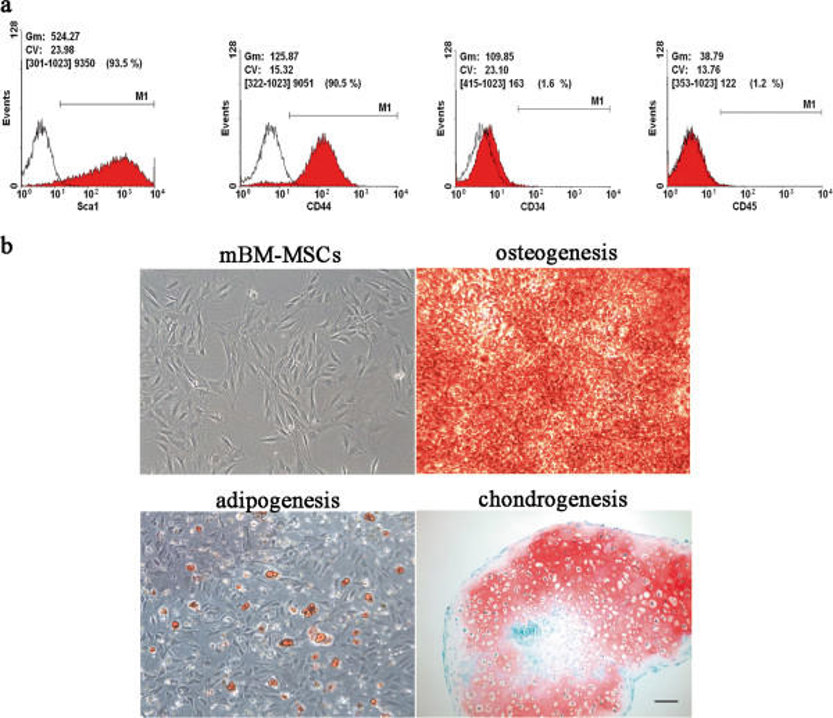
Each batch of Mouse Bone Marrow Mesenchymal Stem Cells are tested for expression of markers using antibodies, CD44, Sca-1 and CD29 by flow cytometry.
Standard biochemical procedures performed with cell cultures include RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining, flow cytometry or generating cell derivatives for desired research applications.
C57BL/6 mouse bone marrow mesenchymal stem cells (BM-MSCs) are multipotent stromal cells derived from the bone marrow of C57BL/6 mice, a commonly used inbred laboratory mouse strain in biomedical research. In culture, these cells are plastic-adherent and exhibit a fibroblast-like morphology. Phenotypically, they express cell surface markers including CD29, CD44, Sca-1, and CD105, while lacking hematopoietic markers such as CD34, CD45, CD11b, and TER-119.
BM-MSCs are known for their strong self-renewal capacity and multilineage differentiation potential, meaning they can differentiate into various cell lineages such as osteoblasts, adipocytes, and chondrocytes under appropriate inductive conditions. They also exhibit immunomodulatory properties, which is, they can suppress immune responses by modulating T cell subsets (e.g., decreasing Th1/Th17 and increasing Th2/Treg) through PD-1/PD-L1. This makes them a useful tool for studying immunomodulatory mechanisms in disease contexts like multiple myeloma, where they are induced and promote tumor progression through inhibiting T-cell mediated immunity. BM-MSCs have applications in regenerative medicine and disease modeling, particularly in bone, cardiovascular, and neurological disorders. In addition, they are commonly used for studying the biology of MSCs, including their proliferation, aging, differentiation, and transplantation behavior. In terms of culturing conditions, isolating and culturing these cells requires optimization due to potential issues such as hematopoietic cell contamination. Like other MSCs, BM-MSCs are sensitive to oxygen levels in their culture environment and studies have shown that culturing these cells under low oxygen (5%) conditions increases their proliferation and clonogenicity compared to normoxia.
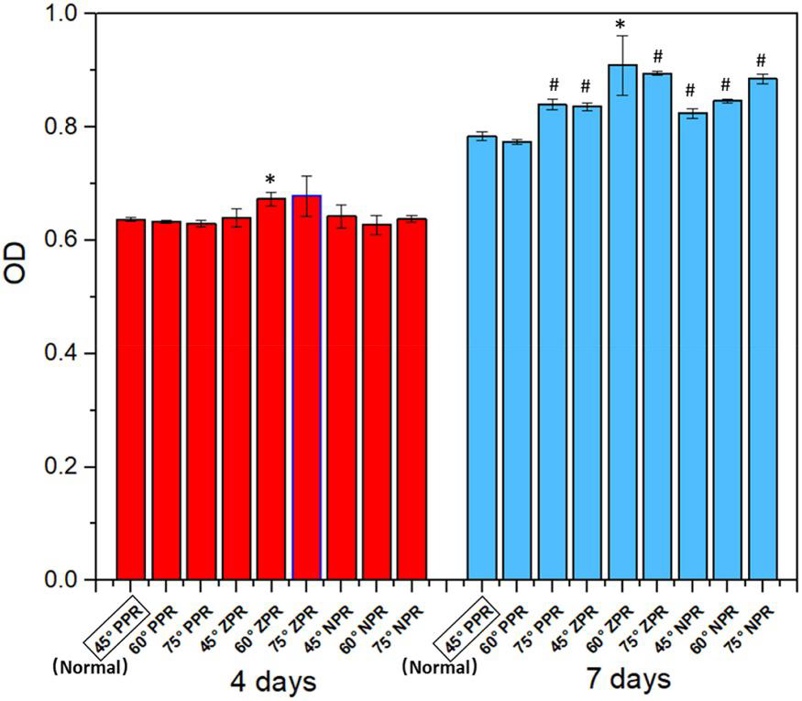
Bone Marrow Mesenchymal Stem Cells Proliferation on the Scaffolds with Different PRs
Tissue engineering aims to create scaffolds that mimic the native tissue environment to support cell survival and growth. Sun's team focuses on developing cellular structured scaffolds with tunable Poisson's ratios (PRs) using a composite material. The objective is to investigate how different PRs affect the proliferation and differentiation of bone marrow mesenchymal stem cells (BMSc) cultured on these scaffolds.
C57BL/6 mouse BMSc were seeded onto the scaffolds at a density of 1×106 cells/ml and cultured in an incubator at 37°C with 5% CO₂, with medium changes every two days. After one week, the medium was switched to chondrogenic induction differentiation medium for another 14 days. Cell-scaffolds were harvested from each group after 4 and 7 days. The growth situation of cells on the different scaffolds is shown in Figure 1. The optical density value represents the formazan content generated by the interaction of the CCK8 reagent with living cells. Higher absorbance values indicate more cells on the scaffold. Results show that cell numbers on all scaffolds were similar in the first 4 days. After 7 days, most groups had significantly more cells than the control group, with cells on the 60° and 75° zero Poisson's ratio (ZPR) scaffolds growing slightly faster. This confirms that PEGDA/CNF aerogel scaffolds with different PRs do not negatively affect BMSc growth.
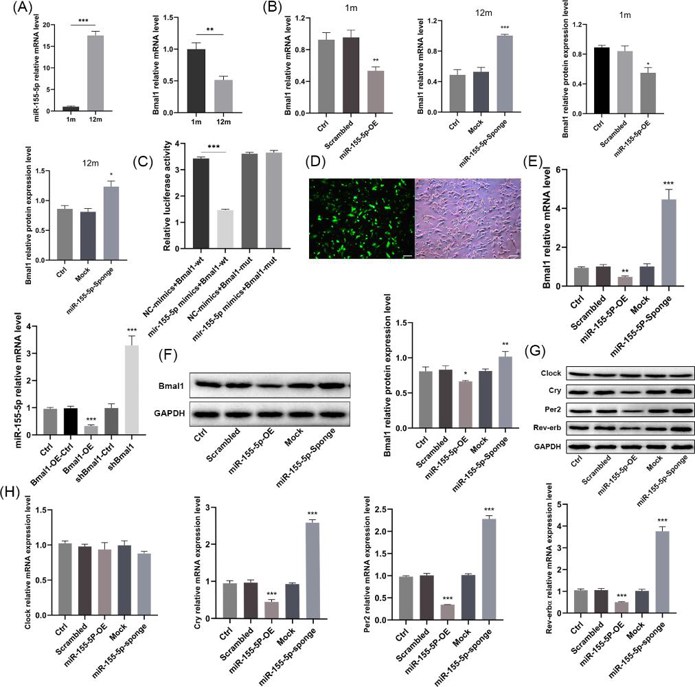
miR-155-5p Targeted and Had a Reciprocal Suppressive Effect with Bmal1, Simultaneously Modulating Other Core Clock Genes
Bone tissue aging is regulated by the core clock gene Bmal1. Current research has established the link between Bmal1 and bone senescence but lacks in-depth exploration of its regulatory mechanisms. Zhang's team aims to investigate the interaction between miR-155-5p and Bmal1 and their effects on the aging and osteogenic differentiation of mouse bone marrow mesenchymal stem cells (BMSCs).
miR-155-5p mRNA expression in BMSCs of old mice was significantly higher than in young mice and the expression of Bmal1 was reduced with aging (Fig. 2a). They used lentivirus to achieve miR-155-5p control over BMSCs. The overexpression of miR-155-5p reduced Bmal1 mRNA and protein levels in the young group, while the inhibition of miR-155-5p increased them in the old group (Fig. 2b). TargetScan and miRanda analysis revealed that miR-155-5p targeted bases 40-47 and 235-241 of Bmal1 3'UTR. The luciferase reporter assay showed that in BMSCs transfected with pSI-Check2-wt-Bmal1, the miR-155-5p mimics significantly reduced the relative fluorescence value compared with the negative control, but no significant difference was observed in the mut-Bmal1 group (Fig. 2c). The miR-155-5p/Bmal1 overexpression/inhibition stable BMSC cell lines were successfully constructed (Fig. 2d). They discovered that overexpression of miR-155-5p downregulated the expression of Bmal1, while knockdown of miR-155-5p upregulated the expression of Bmal1. Bmal1 overexpression reduced miR-155-5p levels, while inhibition of Bmal1 increased miR-155-5p levels, suggesting that they mutually suppressed each other (Fig. 2e, f). There was no significant difference in Clock mRNA expression after miR-155-5p transfection, but it reduced the transcription of Cry, Per2 and Rev-erbα, while inhibition of miR-155-5p increased the expression (Fig. 2g, h).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells