
KYSE220
Cat.No.: CSC-C6799J
Species: Homo sapiens (Human)
Source: Esophagus
Morphology: Epithelial-Like
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
KYSE-220 is a human esophageal squamous cell carcinoma (ESCC) cell line originally created by Kouichi Shimada et al, which is a member of the extensively-phenotyped KYSE cell line panel which was developed from a series of Japanese ESCC patient tumors. KYSE-220 is epithelial-like and exhibits typical cancer cell morphology, forming cohesive, polygonal, monolayered sheets of cells with abundant cell-cell junctions. The cell line displays consistent growth under routine culture conditions (generally RPMI-1640 with 10% FBS) and does not show signs of senescence or crisis through passages.
Genomic features of KYSE-220 include structural and numerical chromosomal aberrations, common in ESCC, and other genetic alterations such as mutations in the tumor suppressor genes TP53 and CDKN2A, as well as amplification of oncogenic 3q. In addition, KYSE-220 expresses established ESCC biomarkers, including EGFR and SOX2. The cell line is capable of many typical ESCC cancer cell functions, such as robust proliferation, invasion, and sensitivity to chemotherapeutic and targeted therapeutics. KYSE-220 has been utilized in numerous ESCC-related tumor biology studies, including, but not limited to, studies of carcinogenesis, oncogenic signaling, epithelial-mesenchymal transition, drug response, and biomarker validation.
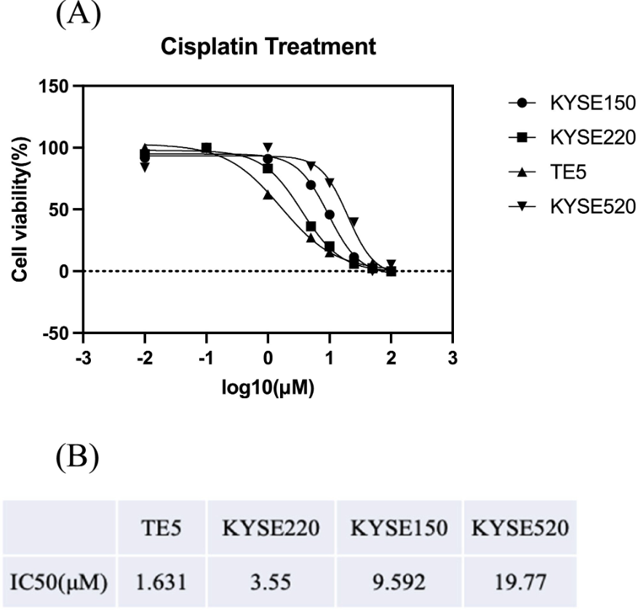
Evaluation of Cisplatin Sensitivity in ESCC Cell Lines Using Cell Proliferation Assay
ESCC is an aggressive cancer with a poor prognosis. Cisplatin-based NAC is the first-line chemotherapeutic drug for ESCC. However, cisplatin resistance is a critical problem in ESCC chemotherapy. In this study, Mozumi et al. investigated LAT1 expression in ESCC patient samples after NAC and analyzed the correlation of LAT1 with clinicopathological factors and patient survival. They also evaluated LAT1 expression in ESCC cell lines with different cisplatin sensitivities, performed radiotracer detection of amino acid metabolism, and conducted RNA sequencing to screen for differentially expressed genes. They performed cell proliferation assays to detect the proliferation of the four ESCC cell lines (TE5, KYSE150, KYSE220, and KYSE520) treated with cisplatin. The cells showed different degrees of sensitivity to cisplatin. The IC50 values for TE5, KYSE220, KYSE150, and KYSE520 were 1.631, 3.55, 9.592, and 19.77 μM, respectively (Fig. 1). TE5 had the highest sensitivity to cisplatin, and KYSE520 was the most resistant to cisplatin.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells





