
Immortalized Human Pulmonary Microvascular Endothelial Cells
Cat.No.: CSC-I9128L
Species: homo sapiens
Source: Lung
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Immortalized Human Pulmonary Microvascular Endothelial Cells (HPMVECs) are human pulmonary microvascular endothelial cells that have been immortalized by genetic engineering. In vivo, pulmonary vascular endothelial cells play many important physiological functions, such as maintaining vascular barrier function, regulating blood flow, and mediating inflammation. After being immortalized by genetic engineering methods, they can be passaged stably in vitro for a long time. HPMVECs show endothelial cell morphology with a cobblestone appearance while expressing markers CD31, CD34, vWF (von Willebrand factor), ICAM-1 and VCAM-1. HPMEC cells also display typical endothelial cell functions, such as uptake of low-density lipoprotein (LDL), tube formation (tubulogenesis), and expression of cell adhesion molecules.
HPMVECs can be used to study the physiological and pathological mechanisms of pulmonary vascular endothelial cells, including inflammatory responses, angiogenesis, thrombosis, and pulmonary arterial hypertension. For example, theys can be used to study the effect of SARS-CoV-2 infection on pulmonary vascular barrier function. They are also used often for drug screening, toxicity testing, and drug delivery research, for example, delivery of nanoparticles to the lung.
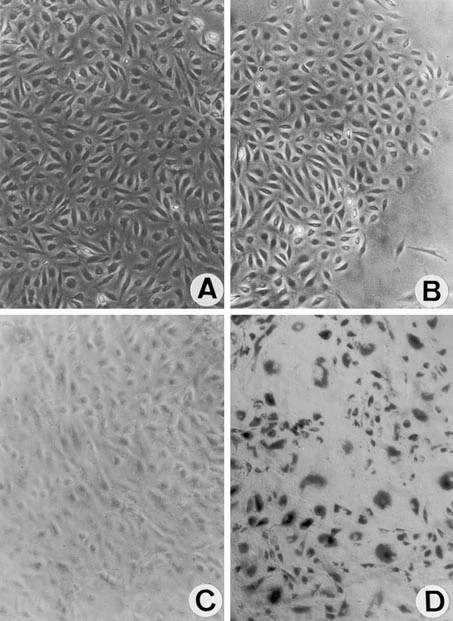 Fig. 1. Human pulmonary microvascular endothelial cells (HPMEC) cultures after cotransfection with plasmids encoding human telomerase reverse transcriptase protein (hTERT) and simian virus 40 (SV40) large T antigen (Krump-Konvalinkova V, Bittinger F, et al., 2001).
Fig. 1. Human pulmonary microvascular endothelial cells (HPMEC) cultures after cotransfection with plasmids encoding human telomerase reverse transcriptase protein (hTERT) and simian virus 40 (SV40) large T antigen (Krump-Konvalinkova V, Bittinger F, et al., 2001).
Endothelial Inflammation as Assessed by ICAM-1, NLRP3, and P-Selectin in the In Vitro HPMVEC COVID-19 Model
Endothelial activation in COVID-19 induces inflammation, however, the mechanism is not well understood. Paul et al. investigated how COVID-19 leads to pulmonary endothelial activation and subsequent pro-inflammatory states. In order to recapitulate systemic inflammation in vitro, human pulmonary microvascular endothelial cells (HPMVEC) were treated with serum from severe COVID-19 patients.
Immune cell adhesion to the vascular wall is driven by inflammation in endothelial cells (EC). To determine the presence of a pro-inflammatory phenotype in HPMVEC, levels of ICAM-1, NLRP3, and P-selectin were quantified in an in vitro model of COVID-19. Cells treated with COVID-19 patient serum had a more robust inflammatory phenotype than those treated with normal human serum. HPMVECs treated with normal serum had low ICAM-1 and NLRP3 expression (green fluorescence) in comparison to COVID-19 serum treated cells (Fig. 1A and C). The fluorescence intensity was quantified using ImageJ, and the total integrated intensity was normalized to the cell area. Figure 1B and 1D present the quantified fluorescence data, with both ICAM-1 and NLRP3 significantly upregulated in the COVID-19 samples. P-selectin, which is upregulated and mediates neutrophil and monocyte binding, was also higher in the COVID-19 group, but not significantly (Fig 1E).
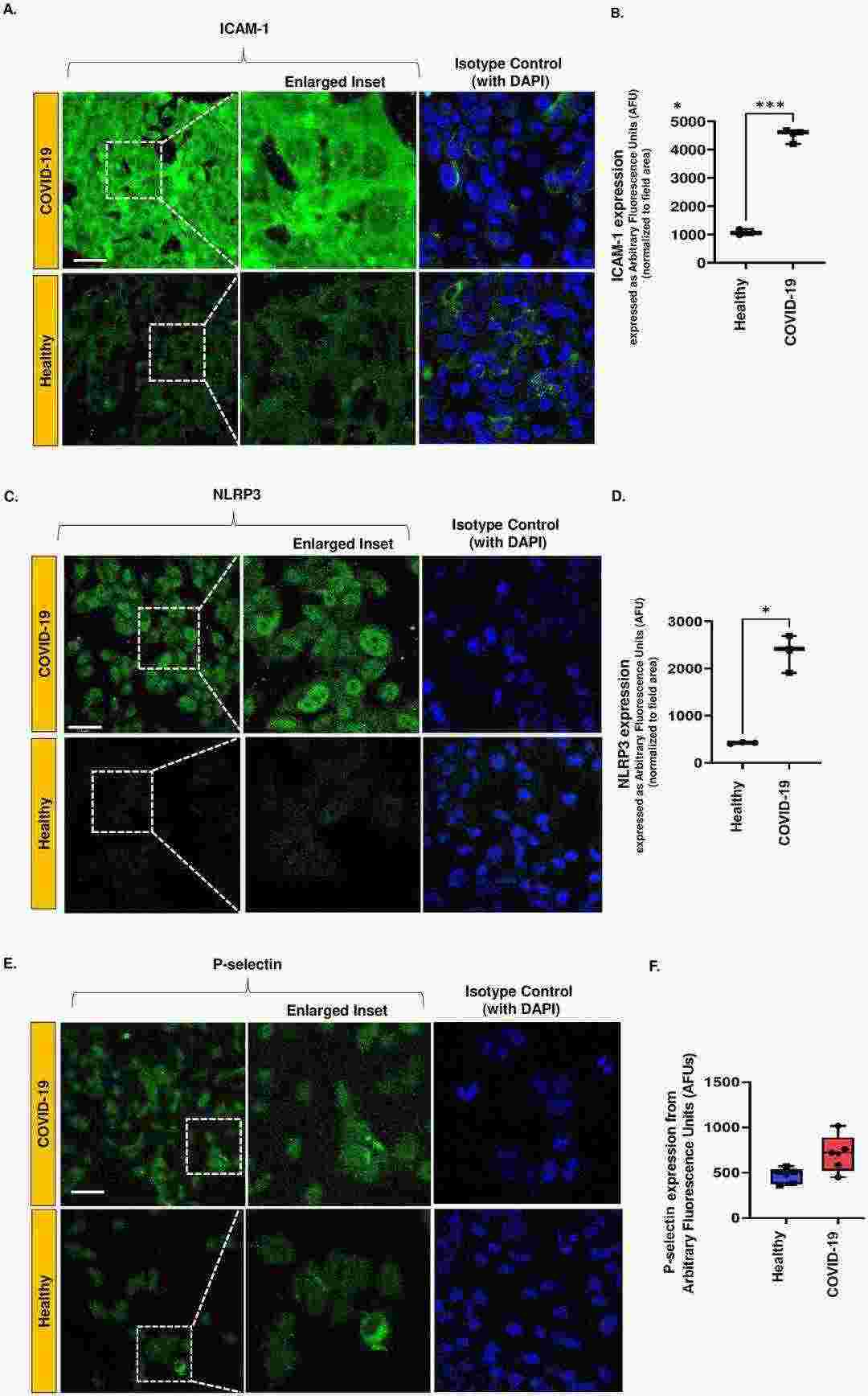 Fig. 1. Expression of inflammatory moieties in an in vitro HPMVEC COVID-19 model (Paul O, Alolia I K, et al., 2024).
Fig. 1. Expression of inflammatory moieties in an in vitro HPMVEC COVID-19 model (Paul O, Alolia I K, et al., 2024).
Nicotine-free E-cigarette Aerosol Triggers Oxidative Stress and ICAM-1 Upregulation in Pulmonary Endothelium
Fine and ultrafine particles in e-cig vapor may induce oxidative stress and endothelial dysfunction, as has been shown for environmental pollutants. Chatterjee et al. determined the acute effects of e-cigarette aerosol inhalation (nicotine-free) on oxidative stress, inflammation, and endothelial activation in healthy nonsmokers by measuring serum biomarkers (CRP, sICAM-1, NOx) and endothelial cell responses (ROS, ICAM-1).
Serum-induced endothelial oxidative stress and inflammation were quantified by assessing ICAM-1 expression (Fig. 2) and ROS production (Fig. 3) in human pulmonary microvascular endothelial cells (HPMVEC) treated with pre- and post-e-cigarette exposure serum. Pre-exposure serum treatment resulted in minimal induction of ICAM-1 expression (barely detectable fluorescence; Fig. 2A), whereas serum collected 120 minutes after exposure caused a significant increase in ICAM-1 (Fig. 2A and B). Images taken at high magnification revealed perinuclear ICAM-1 staining (Fig. 2A, inset). Individual responses varied (subjects S7/S9 had a stronger response); however, ICAM-1 levels returned to baseline by 6 hours after exposure (Fig. 2B). The ICAM-1 response to 120-minute post-exposure serum was significantly elevated (P<0.001 vs baseline).
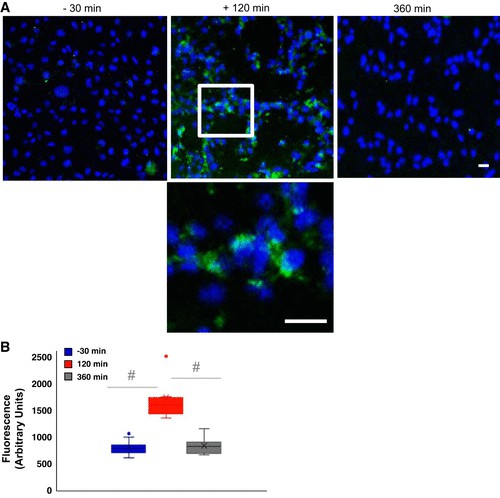 Fig. 2. Effect of serum on endothelial inflammation as monitored by intercellular adhesion molecule (Chatterjee S, Tao J Q, et al., 2019).
Fig. 2. Effect of serum on endothelial inflammation as monitored by intercellular adhesion molecule (Chatterjee S, Tao J Q, et al., 2019).
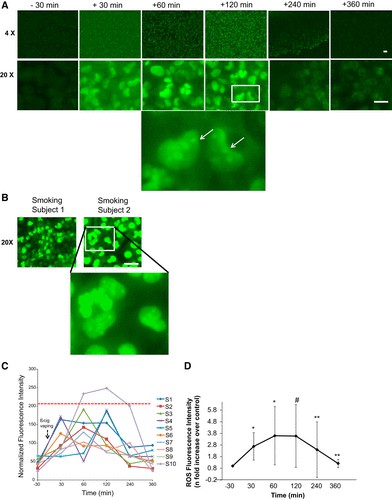 Fig. 3. Endothelial activation potential as monitored by reactive oxygen species (ROS) production by human pulmonary microvascular endothelial cells (HPMVEC) upon treatment with subjects' serum post e-cigarette (e-cig) vaping (Chatterjee S, Tao J Q, et al., 2019).
Fig. 3. Endothelial activation potential as monitored by reactive oxygen species (ROS) production by human pulmonary microvascular endothelial cells (HPMVEC) upon treatment with subjects' serum post e-cigarette (e-cig) vaping (Chatterjee S, Tao J Q, et al., 2019).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
