
Immortalized Human Gastric Epithelial Cells-SV40
Cat.No.: CSC-I2071Z
Species: homo sapiens
Source: Stomach
Cell Type: Epithelial Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Immortalized human gastric epithelial cell line GES-1 was developed from human gastric mucosal tissue. The gastric mucosa serves as the stomach wall's innermost layer that contacts the stomach cavity. Gastric epithelial cells that make up the surface of gastric mucosa produce mucus and gastric acid to protect the mucosa and assist with digestion. Immortalized cell lines show reduced secretory activity compared with primary cells but still serve as valuable tools for studying secretion regulatory mechanisms.
Scientists may use this cell line to study how gastric cancer and related diseases develop. For instance, this cell line acts as a research model to study the effects of Helicobacter pylori on gastric mucosal epithelial cells. Studies have shown that infection with Helicobacter pylori causes GES-1 cells to undergo morphological changes while simultaneously reducing their proliferation and increasing apoptosis rates. By MNNG induction, GES-1 cells can be transformed into a gastric precancerous model for studying the occurrence and development of gastric cancer. Scientists also employ GES-1 cells to test pharmaceutical compounds for their harmful effects on gastric mucosal cellular structures. For example, astragaloside compounds defend against harm in GES-1 cells while Polygonum hydropiper functions to suppress these cells.
H. pylori-infected Macrophage Culture Medium Accelerates Disruption of the Gastric Mucosal Barrier
The Group 1 carcinogen Helicobacter pylori infect more than half of the world's population and causes chronic gastritis by interacting with immune cells in the gastric mucosa. Macrophages that appear at infection sites release inflammatory agents including CCL3 which causes mucosal damage but its pathogenic pathways remain unknown.
Chemokines released by macrophages play vital roles in inflammatory processes. Wei's team investigated how substances released by macrophages infected with H. pylori affect inflammation and damage in gastric mucosa tissue. Both immortalized human gastric epithelial cells (GES-1) and human gastric cancer cell line MKN28 were exposed to supernatant from human leukemia monocytic cells (THP-1) infected with H. pylori. H. pylori-infected macrophage medium (HMC) showed a significant reduction in TEER when compared to normal medium (NC), macrophage medium (MC), and H. pylori medium (HC) (Fig. 1A). The HMC treatment resulted in lower levels of tight junction proteins ZO-1 and Occludin according to the findings in Figures 1B and C. HMC caused disruption of cell tight junctions (Fig. 1D). H. pylori medium was found harmless to gastric mucosa so researchers chose NC and MC as control mediums for further experiments. HMC reduced cell viability and triggered apoptosis (Fig. 1G-H), and inhibited proliferation (Fig. 1I and J). H. pylori-infected macrophage medium leads to increased inflammation in gastric mucosa while breaking down the mucosal barrier.
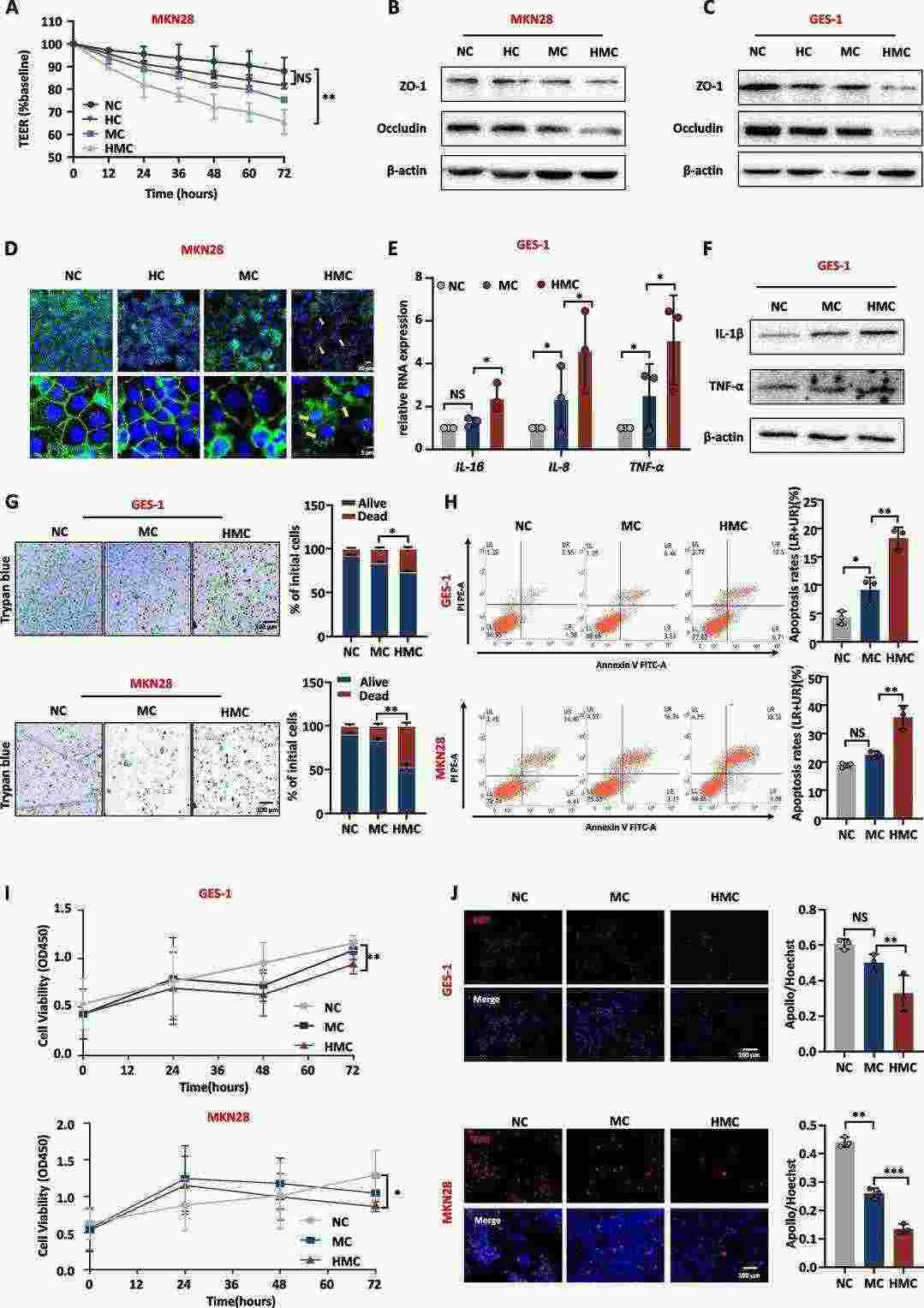 Fig. 1. H. pylori-infected macrophage medium disrupts the gastric mucosa barrier and promotes inflammation (Wei Y F, Li X, et al., 2024).
Fig. 1. H. pylori-infected macrophage medium disrupts the gastric mucosa barrier and promotes inflammation (Wei Y F, Li X, et al., 2024).
NALCN Exerts an Inhibitory Effect on the Biological Function of GC Cells
Research first discovered NALCN during neurological studies before recognizing its important role in cancer metastasis. Li's team seeks to understand NALCN's essential role in gastric cancer development and its influence on immune cell distribution in the tumor microenvironment.
Analysis of RNA sequencing data from fresh frozen tumor and adjacent non-tumor tissues in 22 GC cases revealed that GC patients with low NALCN expression experience worse disease outcomes. The expression of NALCN mRNA and protein showed a significant reduction in GC cells (Fig. 2A). To understand NALCN's role in GC, they initiated three distinct NALCN knockdowns (siNALCN#1, siNALCN#2, siNALCN#3). NALCN expression levels decreased significantly in AGS and GES-1 cells after siRNA knockdown (Fig. 2A and B). Both cell types showed increased cell proliferation, colony formation abilities, and migration after knockdown (Fig. 2D–F). The knockdown of NALCN raised cyclin D1 protein levels while reducing G0/G1 phase cells and increasing G2/M phase cells according to Figure 2G and Figure 2H respectively and led to lower apoptosis rates in AGS and GES-1 cells as seen in Figure 2I. The results demonstrate that NALCN acts as an inhibitor of gastric cancer cell functionality.
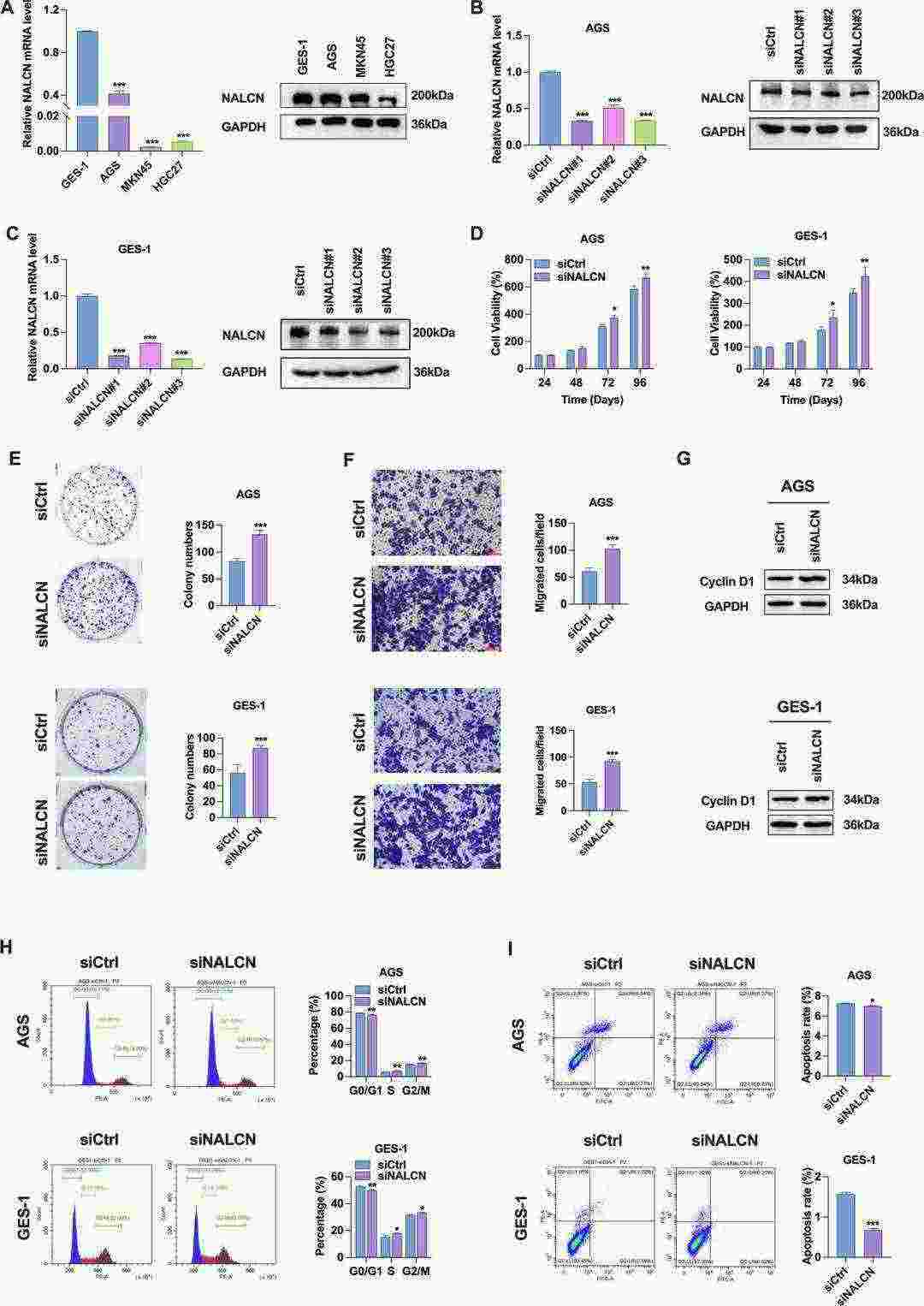 Fig. 2. Effect of NALCN knockdown on biological function of GC cells (Li XW, Wu N, et al., 2025).
Fig. 2. Effect of NALCN knockdown on biological function of GC cells (Li XW, Wu N, et al., 2025).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

