Human Stomach Epithelial Cells
Cat.No.: CSC-C9230J
Species: Human
Source: Stomach
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The source of human stomach epithelial cells is the gastric mucosal tissue. The gastric mucosa represents the innermost stomach wall layer as a single layer of columnar epithelial cells which organize neatly and mainly exist in the stomach body and antrum. The main function of gastric epithelial cells includes the production of digestive enzymes like pepsinogen, hydrochloric acid and protective mucus. The secretions produced by gastric epithelial cells help break down food and shield the stomach lining from acid damage. Gastric epithelial cells defend against infections by producing antimicrobial peptides which block bacterial growth.
Gastric epithelial cells provide essential insights for scientific investigations focused on cancer research and gastrointestinal disease mechanisms. The dysfunction of gastric stem cells directly impacts gastric cancer development thus understanding their molecular regulatory mechanisms will help create novel therapeutic approaches. Additionally, gastric epithelial cells are used to research the interactions between the gastrointestinal microbiota and the host genome, offering new perspectives on understanding the intestinal microecology.
PHLDA1 Decreases Gastric Cancer Cell Survival and Metastasis
PHLDA1 acts as a tumor suppressor gene in gastric cancer yet researchers have yet to determine how its regulation is affected by circular RNAs (circRNAs). While circRNAs play significant regulatory roles in cancer development they remain poorly understood in the context of gastric cancer molecular pathways. Wang's team examined how circular RNAs regulate PHLDA1 expression in gastric cancer through this study.
They investigated PHLDA1 expression levels in SGC-7901, MGC-803, HGC-27, MKN-45, MKN-28, and human stomach epithelial cells gastric cancer cells to determine its role in cell survival. PHLDA1 expression levels were reduced in MKN-28 and HGC-27 cell lines compared to the other examined cell lines (Fig. 1a). They selected these two lines for additional examination. Overexpression of PHLDA1 caused decreased proliferation in both MKN-28 and HGC-27 cells as shown in Figure 1b and 1c. The overexpression of PHLDA1 in cell lines MKN-28 and HGC-27 led to significantly reduced migration abilities (Fig. 1d). Transwell assay results demonstrated reduced cell invasion following PHLDA1 overexpression (Fig. 1e).
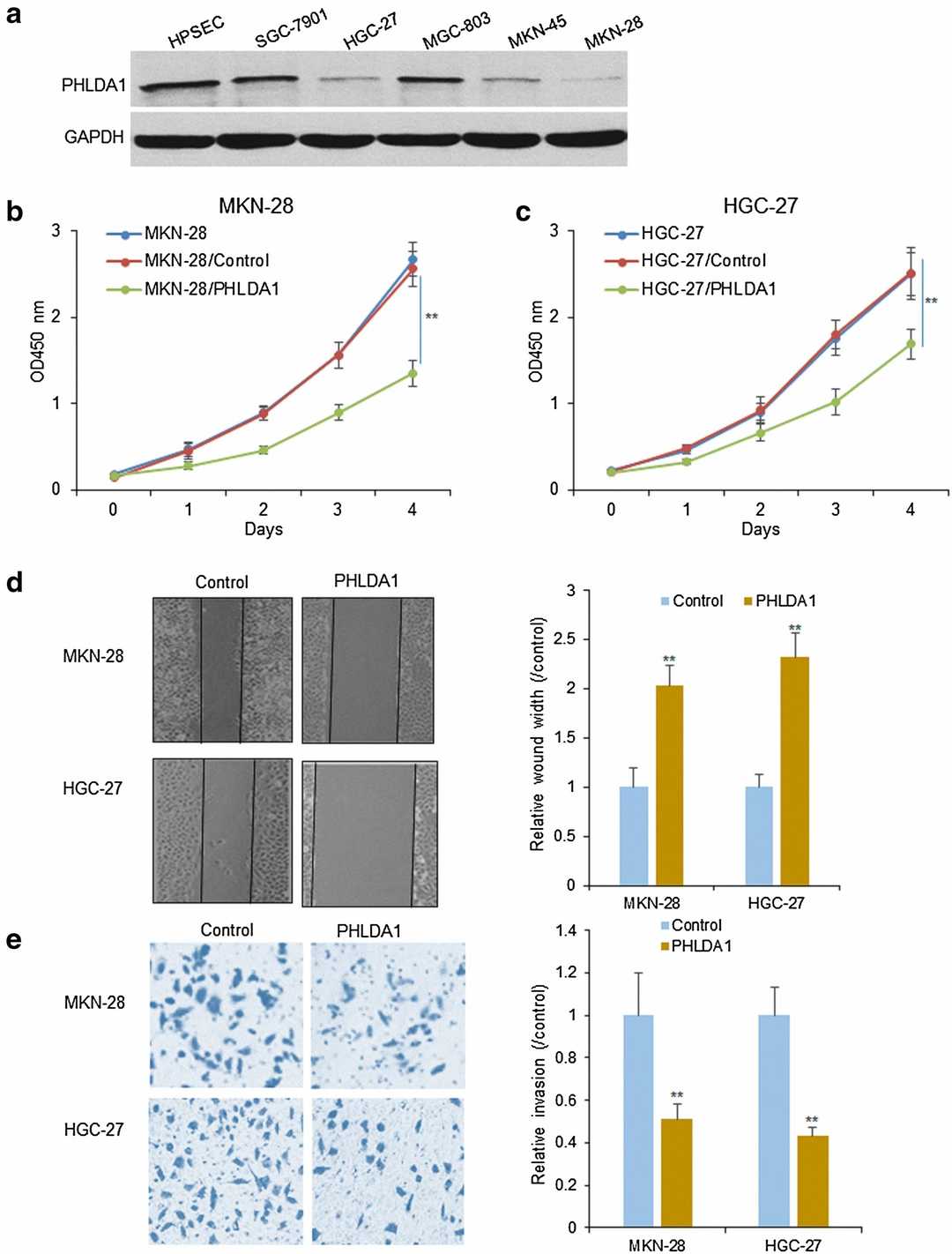 Fig. 1. PHLDA1 decreases gastric cancer cell survival and metastasis (Wang L, Shen JY, et al., 2018).
Fig. 1. PHLDA1 decreases gastric cancer cell survival and metastasis (Wang L, Shen JY, et al., 2018).
The Volatilomic Footprints of Human HGC-27 and CLS-145 Gastric Cancer Cell Lines
Gastric cancer is a leading cause of cancer-related deaths globally, with volatile biomarkers in breath potentially aiding noninvasive diagnosis. While volatile compounds have been associated with gastric cancer, their origins remain unclear. Leiherer et al. elucidated these origins by mapping the volatilomic signatures of HGC-27 and CLS-145 gastric cancer cell lines compared to normal human stomach epithelial cells (HSEC).
Among the detected volatiles, 27 showed notable concentration differences compared to only the cultivation medium. Twelve compounds had reduced headspace concentrations, while others increased. Compounds with increased concentrations (release) include three esters, seven ketones, three alcohols, one aromatic, and one sulfur compound. Compounds absorbed (uptake) comprise eight aldehydes, three heterocyclic compounds, and one sulfur compound. Efforts to quantify these species were made, but three compounds were measured only by peak areas due to issues with substance availability or reliable reference mixtures. To compare the emissions of VOCs by the cells, the associated signal intensities were normalized to the number of cells in particular cultures. The comparison of this emission is presented in Figure 2.
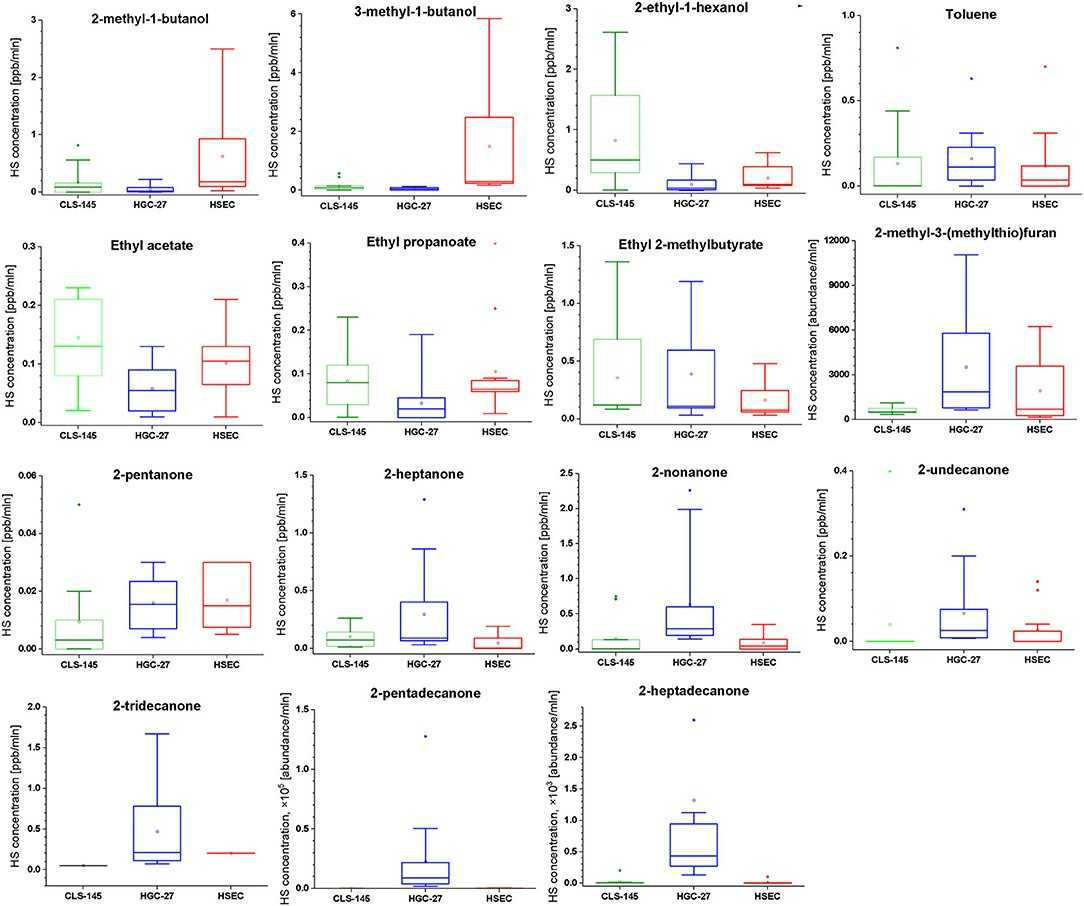 Fig. 2. Comparison of the headspace concentrations of VOCs of interest over the cultures of HGC-27, CLS-145, and HSEC cells (Leiherer A, ?lefarska D, et al., 2020).
Fig. 2. Comparison of the headspace concentrations of VOCs of interest over the cultures of HGC-27, CLS-145, and HSEC cells (Leiherer A, ?lefarska D, et al., 2020).
Ask a Question
Write your own review