Canine Kidney Epithelial Cells
Cat.No.: CSC-C9117J
Species: Dog
Source: Kidney
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Canine Kidney Epithelial Cells, best exemplified by the MDCK (Madin-Darby Canine Kidney) cell line, are immortalized epithelial cells isolated from the renal cortex (proximal convoluted tubules) of a healthy adult female Beagle in 1958. It is by far the most commonly used cell line representing canine renal epithelium, which has largely preserved the basic physiological functions of renal tubule epithelia, making it extremely useful in a wide variety of research applications. Morphologically, like most epithelial cells, these cells have a polygonal/cuboidal shape, clear boundaries between cells, and in the confluent state form a "pavement-like" monolayer in culture.
Functionally, the cell line recapitulates many of the functions of a renal proximal tubule: the selective transport of glucose/ions, metabolism of many drugs through CYP450 enzymes, and the ability to form a high-resistance epithelial barrier (TEER 200-500 Ω·cm² for MDCK-II). They are also highly permissive to a number of viruses, such as influenza and canine coronavirus. Scientifically, they are used to study renal transport mechanisms (e.g., screening SGLT2 inhibitors), model renal fibrosis (via TGF-β-induced EMT), culture influenza viruses for vaccine production, and assess drug nephrotoxicity. Subtypes such as MDCK-II are the go-to cell lines for studies involving barrier function, whereas primary cRPTE cells are more suitable for short-term studies of renal epithelial physiology.

CHV-1 is Endocytosed into MDCK Cells
Canid herpesvirus 1 (CHV-1) is a Varicellovirus which is responsible for self-limiting infections in adult dogs, as well as morbidity and mortality in puppies. Elsa et al. applied a multipronged approach to determine the CHV-1 entry pathway into Madin-Darby canine kidney (MDCK) epithelial cells.
To gain insight into the early events of CHV-1 infection, they used transmission electron microscopy (TEM) to analyze CHV-1 entry into an epithelial cell line. CHV-1 strain CHV-V777 was incubated with MDCK cells at MOI 2.5 at 4°C for 2 hr to allow binding, then moved to a 37°C incubator for 30 sec or 2 min to allow viral entry. Cells were then fixed and processed for TEM analysis to visualize subcellular structures associated with the viral particles immediately after entry. They detected enveloped viral particles within large, irregularly shaped cytoplasmic vesicles at both 30 s and 2 min post-entry (Fig. 1a). The vesicles, which varied from 264 to 1943 nm in diameter, lacked a cytoplasmic coat (Fig. 1b). They detected a single occurrence of a naked capsid in the cytoplasm early in infection, which supported an endocytic entry mechanism for CHV-1. Additionally, external viral particles were often detected adjacent to plasma membrane protrusions (Fig. 1c), leading them to hypothesize that CHV-1 enters MDCK cells via macropinocytosis.

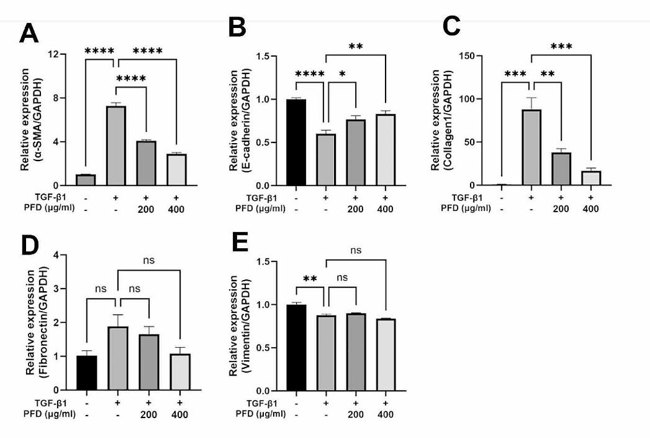
Pirfenidone Inhibits TGF-β1-induced Gene Expression of EMT Markers and ECM Components
Incidence of CKD in dogs is higher in old age and renal fibrosis is one of the underlying mechanisms. However, there are limited drugs that can inhibit fibrosis in the kidney of dogs. The purpose of this study was to test if pirfenidone, a drug with known antifibrotic effects on human cells, also exerts its action on canine renal tubular epithelial cells (MDCK).
The effect of TGF-β1 on mRNA expression of EMT markers associated with fibrosis and ECM components in MDCK cells and pirfenidone on these expressions was examined. TGF-β1 significantly upregulated the expression of α-SMA (Fig. 2A) and downregulated E-cadherin (Fig. 2B) and these trends were reversed by pirfenidone in a dose dependent manner. Expression of collagen1 which was increased by TGF-β1, was inhibited in a dose dependent manner by pirfenidone (Fig. 2C). Neither TGF-β1 stimulation nor pirfenidone treatment led to significant changes in fibronectin expression (Fig. 2D). At 12, 24, and 48 h, mRNA expression of fibronectin was increased with TGF-β1, while pirfenidone treatment did not show consistent change. Expression of vimentin was decreased by TGF-β1 stimulation and pirfenidone treatment did not significantly alter its expression (Fig. 2E). Pirfenidone is able to regulate TGF-β1-induced changes of ECM components and EMT markers at the transcription level.
Ask a Question
Write your own review