Rat Umbilical Vein Endothelial Cells
Cat.No.: CSC-C5077S
Species: Rat
Source: Umbilical Cord; Vein
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Umbilical Vein Endothelial Cells from Creative Bioarray are isolated from the rat umbilical cord tissue. The method we use to isolate Rat Umbilical Vein Endothelial Cells was developed based on a combination of established and our proprietary methods. The Rat Umbilical Vein Endothelial Cells are characterized by immunofluorescence with antibodies specific to PECAM-1/CD31 or von Willebrand factor (vWF). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Researchers obtain rat umbilical vein endothelial cells from the umbilical vein tissue of experimental rats. The umbilical vein functions as a vital connection between the fetus and mother since it transports fetal metabolic waste and excess nutrients to the mother for metabolic processing. These cells perform essential functions in vascular physiology such as maintaining vascular homeostasis and regulating thrombosis as well as involvement in inflammation and angiogenesis promotion. The secretion of cytokines and growth factors including vascular endothelial growth factor (VEGF), platelet-endothelial cell adhesion molecule (PECAM-1/CD31), and von Willebrand factor (vWF) by these cells plays a crucial role in angiogenesis and repair mechanisms.
Rat umbilical vein endothelial cells serve as key research tools for vascular biology and cardiovascular disease studies because they are readily available and easy to isolate and culture. For example:
Cardiovascular disease research: Researchers utilize these cells to examine conditions like vascular sclerosis, atherosclerosis, and thrombosis within cardiovascular disease studies. The simulation of cellular behaviors across various conditions allows scientists to investigate disease processes and develop therapeutic approaches.
Drug screening: The assessment of drug effects on endothelial cell activities targets regulation aspects such as angiogenesis processes, vascular contraction mechanisms and inflammatory response pathways.
Tissue engineering: Tissue engineering products such as artificial blood vessels are created using rat umbilical vein endothelial cells which promote tissue regeneration and repair.
SrHAW@PDA Improved the Biological Performance of BMSCs and UVECs
Hydroxyapatite nanowires (HAW) can effectively improve the bone repair ability in bone engineered tissue. However, due to their single function, the application of HAWs in biological tissue engineering materials is limited. In this study, strontium-doped hydroxyapatite nanowires (SrHAW) were synthesized by a hydrothermal method and coated with polydopamine (PDA) to improve the function of HAWs.
By co-culturing these with rat BMSCs and UVECs, Li's team aims to assess how strontium doping and PDA coating enhance cell activity, osteogenesis, and angiogenesis. To assess the biotoxicity of nanowires on BMSCs and UVECs, co-cultures were done with sets of HAW, 4SrHAW, and 4SrHAW@PDA. After 7 days, Live/Dead staining showed that BMSCs exhibited cell aggregation in the HAW and 4SrHAW groups, especially lacking Sr. In contrast, 4SrHAW@PDA cells grew healthily in a whirlpool-like pattern (Fig. 1A). The CCK-8 assay indicated a slow growth rate in the HAW group, while the others were faster, aligning with the staining results. Cell viability was highest with 4SrHAW@PDA, but differences with 4SrHAW were insignificant. Although UVEC viability was similar across groups, it was slightly higher with 4SrHAW@PDA, indicating improved biocompatibility due to PDA treatment (Fig. 1B and C).
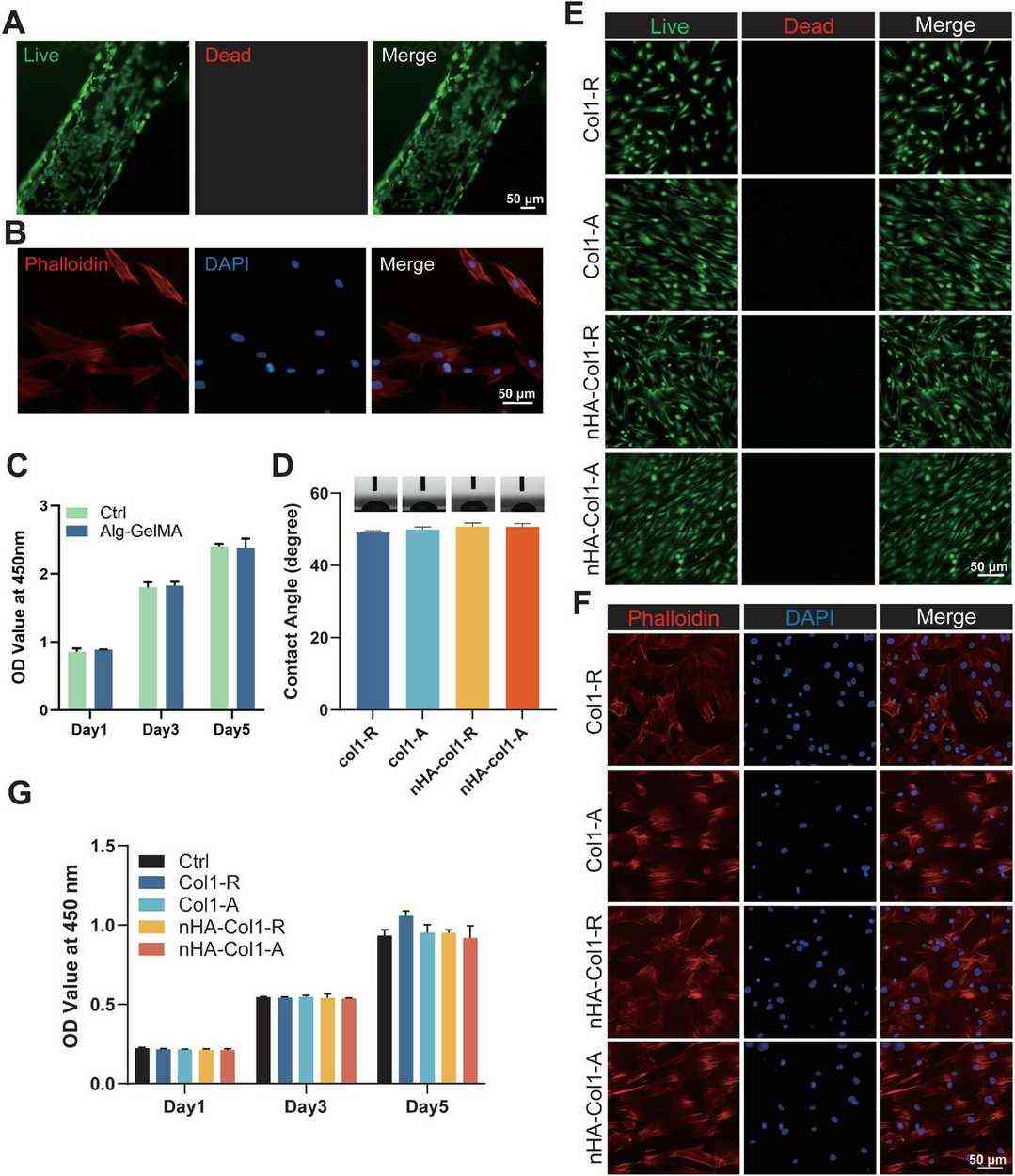 Fig. 1. Biocompatibility of HAW, 4SrHAW, 4SrHAW@PDA nanowires (Li H, Liu Y, et al., 2025).
Fig. 1. Biocompatibility of HAW, 4SrHAW, 4SrHAW@PDA nanowires (Li H, Liu Y, et al., 2025).
Cell Growth on the RUVEC-BMVs and Nanofibrous Membranes
Bone regeneration faces challenges, particularly with large defects and pathologies. Although autologous bone grafting is the gold standard, it is limited by availability and quality. Biomimetic materials offer promising solutions. Zhu's team aims to create a functional 3D biomimetic osteon, replicating the structure and function of natural bone tissue to enhance regeneration, using electrospun nanofibers and microfluidically constructed blood vessels to emulate osteons fully. The biomimetic blood vessels, seeded with rat umbilical vein endothelial cells, secreted bone morphogenetic protein to stimulate osteogenesis and released platelet-derived growth factor to promote angiogenesis, further supporting osteogenesis processes.
Biocompatibility is crucial for biomaterials used in implants. After five days, over 95% of RUVECs in RUVEC-BMVs were viable, showing excellent compatibility (Fig. 2A). To assess Alg-GelMA hydrogels, BMSCs were cult.d on them, and after one day, they showed good adhesion and spreading (Fig. 2B). BMSC viability was similar on Alg-GelMA hydrogels and standard plates, confirming compatibility (Fig. 2C). Nanofibrous scaffold hydrophilicity was evaluated by water contact angle; type I collagen scaffolds had a ≈ 50° contact angle. Adding nHA didn't alter this, indicating consistent hydrophilicity (Fig. 2D). To assess the biocompatibility of four nanofibrous scaffolds (Col 1-R, Col 1-A, nHA-Col 1-R, nHA-Col 1-A), BMSCs were cultured on them. After two days, live/dead and cytoskeletal staining showed most cells were viable with few dead (Fig. 2E and F). BMSCs on random fiber scaffolds (Col 1-R, nHA-Col 1-R) grew irregularly, while those on aligned scaffolds (Col 1-A, nHA-Col 1-A) had an organized pattern. Cytoskeletal staining confirmed well-spread cells on all scaffolds. The CCK-8 assay over 1, 3, and 5 days showed no significant differences in cell viability among scaffolds and the control, indicating similar biocompatibility (Fig. 2G).
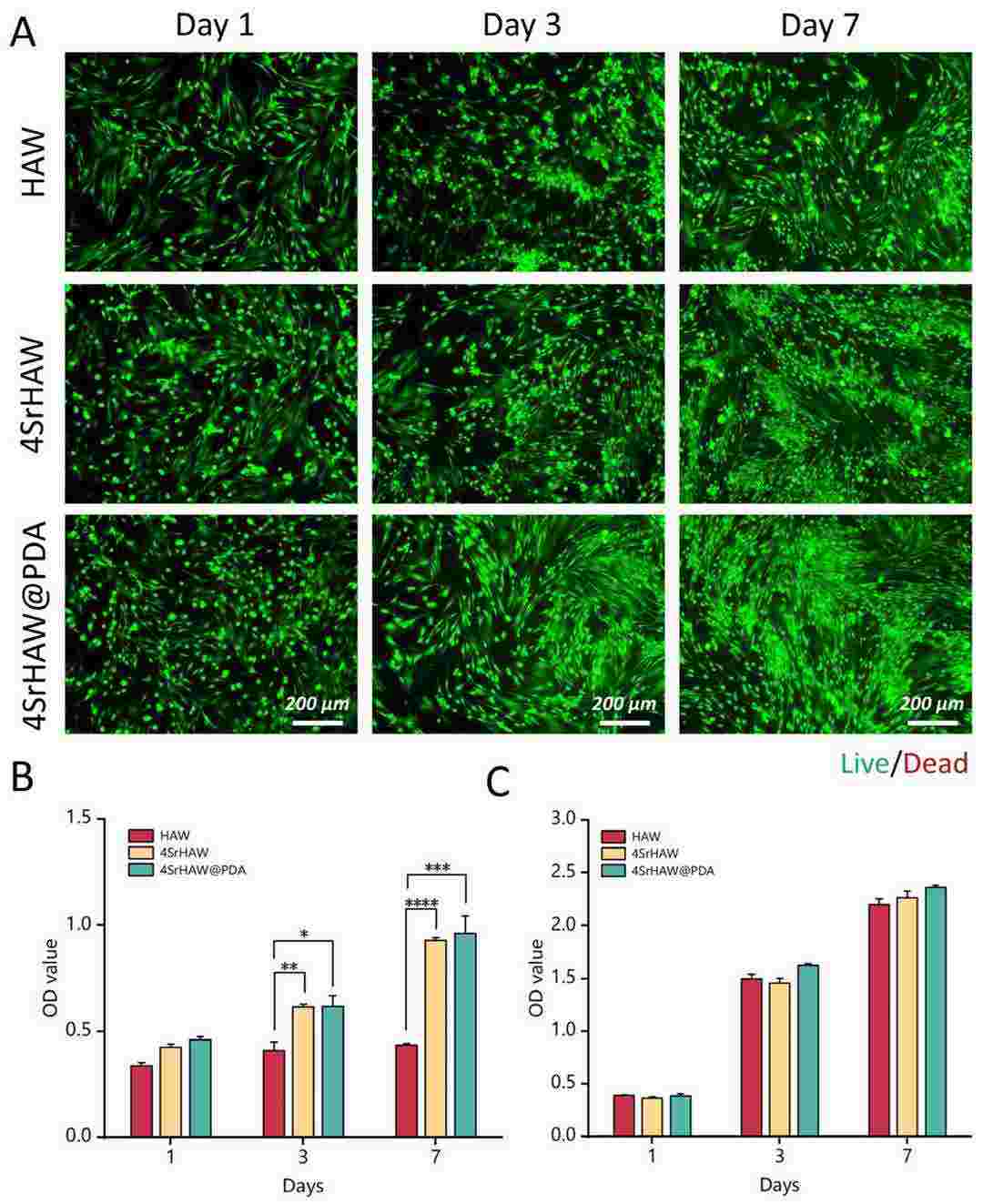 Fig. 2. Cell growth on the RUVEC-BMVs and nanofibrous scaffolds (Zhu CX, Li ZX, et al., 2025).
Fig. 2. Cell growth on the RUVEC-BMVs and nanofibrous scaffolds (Zhu CX, Li ZX, et al., 2025).
Ask a Question
Write your own review
- You May Also Need