Rat Retinal Ganglion Cells
Cat.No.: CSC-C5075S
Species: Rat
Source: Retina; Eye
Cell Type: Retinal Ganglion Cell; Neuron
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Retinal Ganglion Cells (rRGCs) from Creative Bioarray are isolated from the rat retinal tissue. The method we use to isolate rRGCs was developed based on a combination of established and our proprietary methods. The rRGCs are characterized by immunofluorescence with antibodies specific to MAP-2. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Retinal RGCs originate from the ganglion cell layer which contains multipolar neurons positioned at the retina's deepest point. The dendrites of RGCs connect with bipolar and amacrine cells and their axons extend to the optic nerve head before passing through the lamina cribrosa to create the optic nerve which transmits visual information to the brain. About two-thirds of retinal neurons are RGCs which makes them crucial for retinal function. Retinal ganglion cells send their axons primarily to the suprachiasmatic nucleus (SCN), the central nervous system component that controls circadian rhythms in adult rat models. Furthermore, RGCs possess multiple neurotransmitters and ion channels such as NMDA receptors, AMPA/kainate receptors and GABA receptors which facilitate their synaptic transmission and excitability. RGC functionality depends on calcium ion activity since elevated calcium levels stimulate neurotransmitter discharge.
RGCs play a fundamental role in research related to retinal diseases. Programmed cell death in RGCs has significant links with medical conditions which include glaucoma, retinopathy and neurodegenerative diseases. The study of RGCs' regenerative capabilities allows researchers to develop new treatments through stem cell transplantation and gene therapy. The patch-clamp technique enables researchers to study RGCs' electrical properties to understand their role in transmitting visual signals.
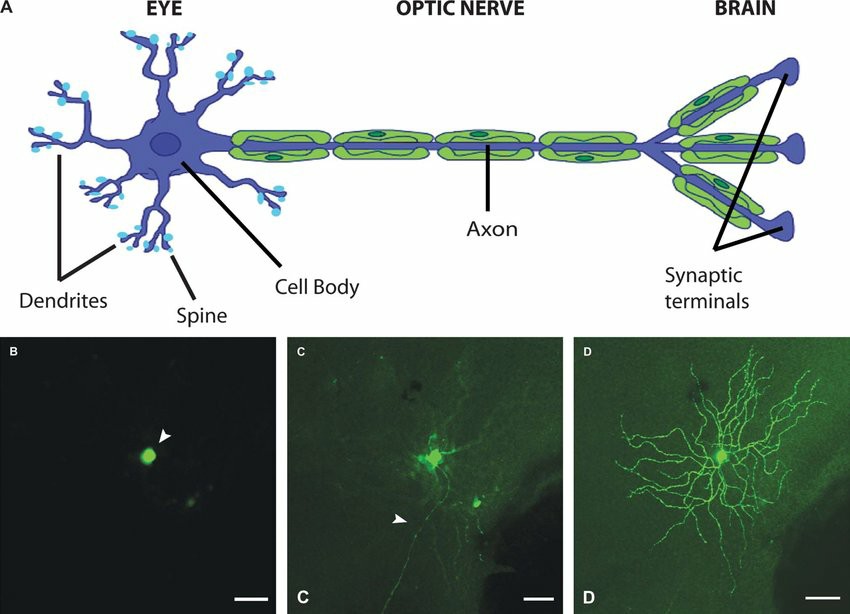 Fig. 1. (A) Retinal ganglion cells: dendrites, cell body, axon, and synaptic terminals. (B-D) Confocal images of different cellular compartments of an adult mouse RGC expressing yellow fluorescent protein (Morquette B J and Polo A D, 2008).
Fig. 1. (A) Retinal ganglion cells: dendrites, cell body, axon, and synaptic terminals. (B-D) Confocal images of different cellular compartments of an adult mouse RGC expressing yellow fluorescent protein (Morquette B J and Polo A D, 2008).
The Expression and Function of TRPV4 in Retinal Ganglion Cells at Elevated Pressure
The study investigated TRPV4 expression levels in retinal ganglion cells (RGCs) under different pressures using immunofluorescence and western blotting techniques together with TUNEL assays. Wang's team investigated TRPV4 channels, which play a role in human senses and might be involved in glaucoma-induced retinal damage.
Using immunofluorescence, western blotting, and TUNEL assays, TRPV4 expression in retinal ganglion cells (RGCs) under varying pressures was examined. Western blotting showed reduced TRPV4 levels at 40 mmHg and 60 mmHg for 24 hours compared to normal culture conditions (Fig. 1A-D). TRPV4 levels increased in GRCs at these pressures. Pathway enrichment scores for glaucomatous cells were analyzed using the GSEA algorithm to find correlations between samples and pathways (Fig. 1E-G). TRPV4 expression correlated positively with apoptosis, PI3K-AKT-mTOR, and ROS-upregulated pathways. These pathways are also linked to apoptosis in cancer, rheumatoid arthritis, and acute respiratory conditions.
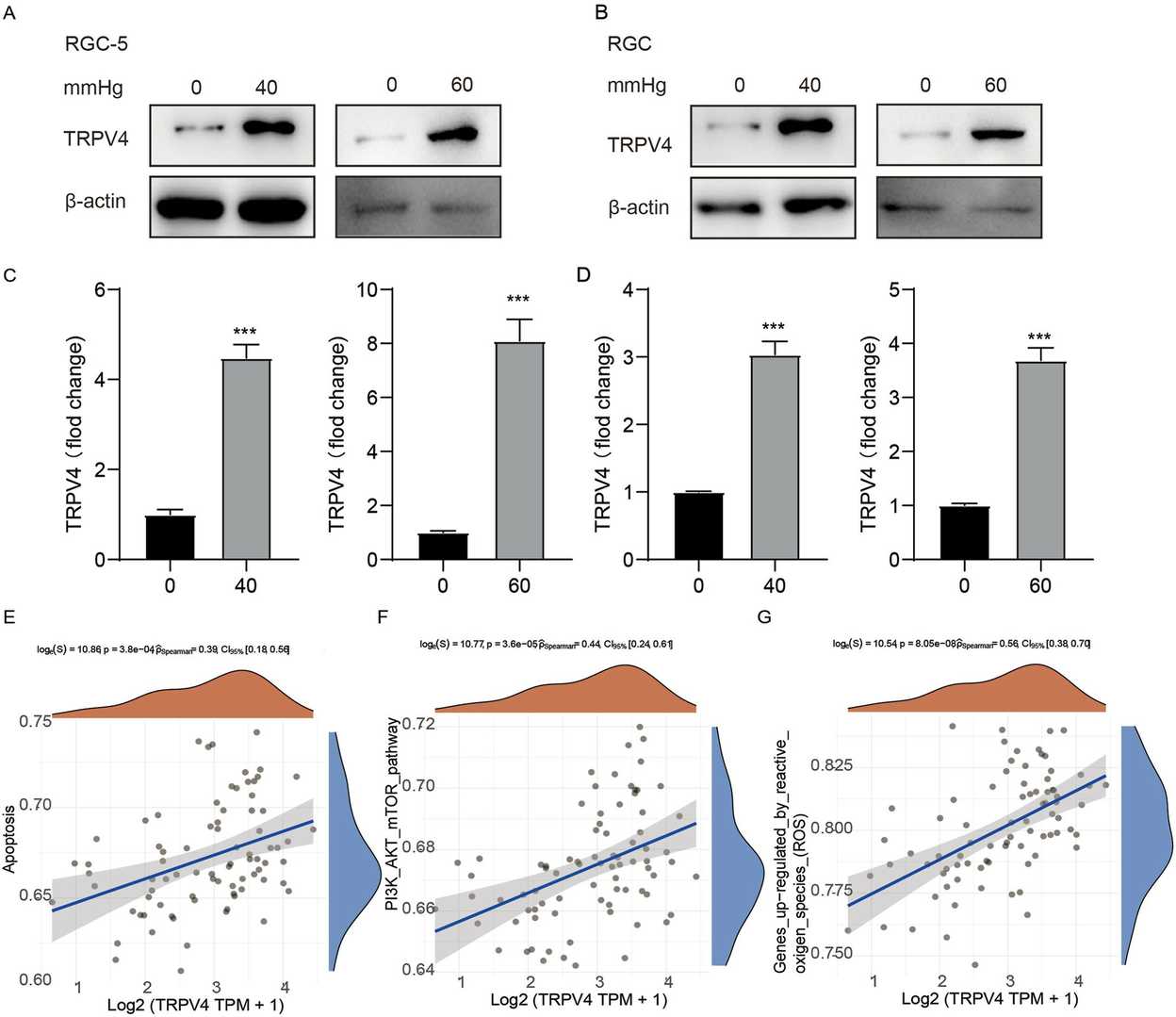 Fig. 1. TRPV4 expression levels in RGCs in the glaucoma model (Wang L, Zhang W, et al., 2023).
Fig. 1. TRPV4 expression levels in RGCs in the glaucoma model (Wang L, Zhang W, et al., 2023).
SA-2 Is Neuroprotective to Primary RGCs in the Trophic Factor Deprivation Model
Glaucoma is characterized by degeneration of retinal ganglion cells (RGCs), resulting in optic nerve damage and vision loss. Current treatments largely focus on reducing intraocular pressure (IOP) and do not prevent RGC loss. These neurons, essential for transmitting visual data, are vulnerable to oxidative damage due to high metabolic demands and insufficient antioxidant responses, leading to apoptosis.
Neurotrophic factors are crucial for RGC survival. Pham et al. modeled RGC death by depriving these factors, treating primary RGCs with SA-2 at 0.1 mM, 0.5 mM, and 1 mM for 48 hours. Apoptotic and dead cells were stained with LIVE Green Caspase-3 and -7 Detection Kit. TF deprivation resulted in a 37.83% decrease in RGCs. SA-2 significantly increased RGC preservation across all concentrations (0.1 mM: 108.87% increase, p < 0.01; 0.5 mM: 158.93%, p < 0.001; 1 mM: 74.96%, p < 0.01) compared to vehicle (DPBS) (Fig. 2A-B, teal color). Apoptotic RGCs decreased significantly at 0.1 mM (93.72%), 0.5 mM (99.99%), and 1 mM (92.78%) (Fig. 2A-B, green color). Dead RGCs also reduced significantly at all concentrations (Fig. 2A-B, orange color). In the presence of neurotrophic factors, SA-2 significantly increased RGCs at concentrations of 0.5 mM (232.45% increase, p < 0.0001) and 1 mM (194.74%, p < 0.0001) (Fig. 3A-B, teal). Primary RGCs naturally die even with neurotrophic factors, but SA-2 reduced apoptotic cells at 0.1 mM (52.5% decrease, p < 0.0001), 0.5 mM (30%, p < 0.01), and 1 mM (52.48%, p < 0.001) (Fig. 3A-B, green). SA-2 also decreased dead RGCs at 0.1 mM (95.33% decrease, p < 0.0001) and 0.5 mM (99.99%, p < 0.0001) compared to control (Fig. 3A-B, orange). These findings demonstrate SA-2's potential to protect RGCs from apoptosis within the TF deprivation model.
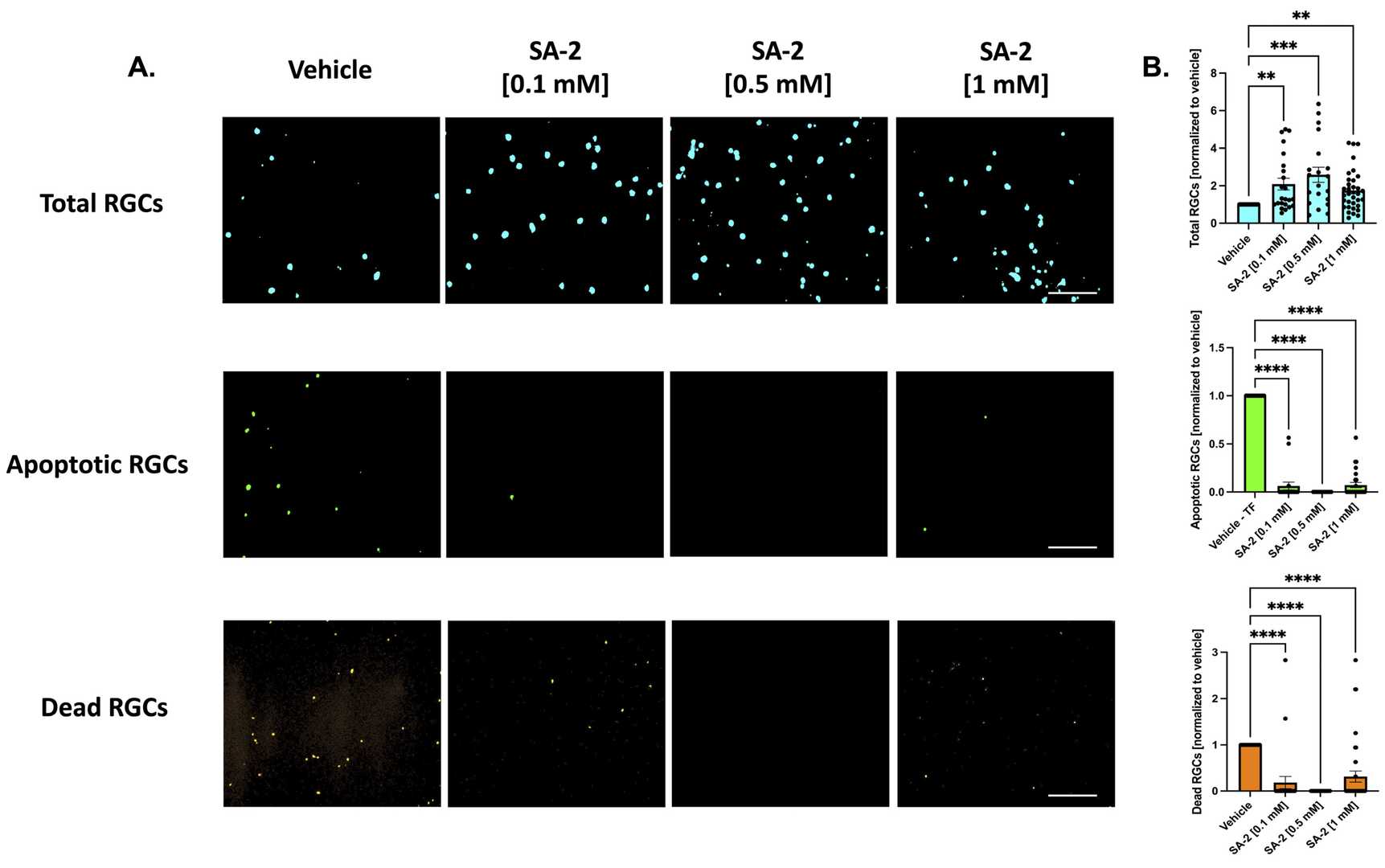 Fig. 2. Treatment of primary retinal ganglion cells (RGCs) from Sprague Dawley rat pups in the absence of neurotrophic factors (Pham J H, Johnson G A, et al., 2022).
Fig. 2. Treatment of primary retinal ganglion cells (RGCs) from Sprague Dawley rat pups in the absence of neurotrophic factors (Pham J H, Johnson G A, et al., 2022).
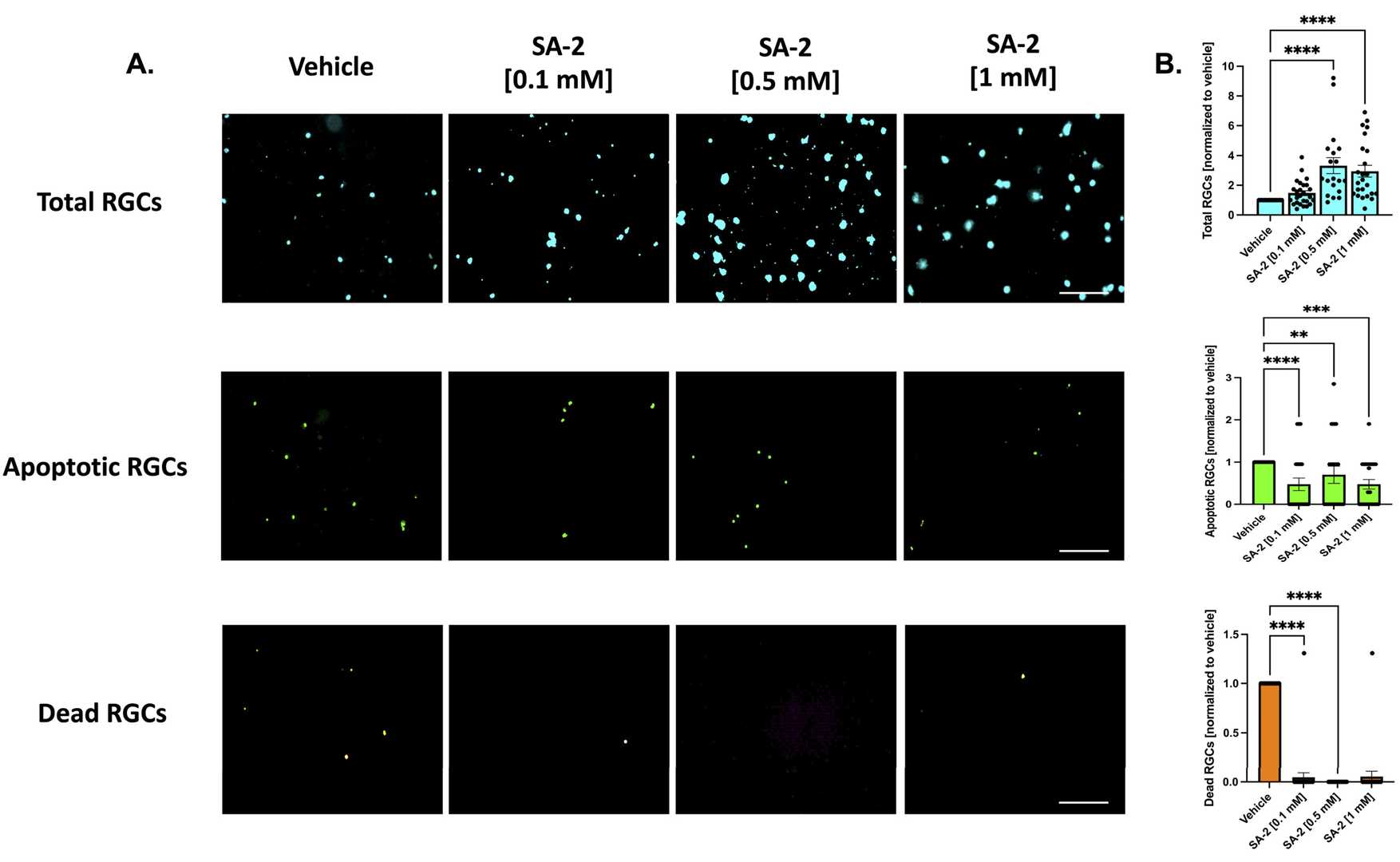 Fig. 3. Treatment of primary RGCs from Sprague Dawley rat pups in the presence of neurotrophic factors (Pham J H, Johnson G A, et al., 2022).
Fig. 3. Treatment of primary RGCs from Sprague Dawley rat pups in the presence of neurotrophic factors (Pham J H, Johnson G A, et al., 2022).
Ask a Question
Write your own review