- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Parathyroid Cells from Creative Bioarray are isolated from the rat parathyroid tissue. The method we use to isolate Rat Parathyroid Cells was developed based on a combination of established and our proprietary methods. The Rat Parathyroid Cells are characterized by immunofluorescence with antibodies specific to parathormone (PTH). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rat parathyroid cells are endocrine cells derived mainly from the parathyroid glands, which are four small ovoid endocrine glands located on the back (posterior) surface of the rat thyroid gland, next to the thyroid follicles. The cells are polygonal or epithelial-like in vitro, with a round centrally located nucleus, abundant cytoplasm, and intercellular junctions consistent with their endocrine and glandular origin. The cells are adherent in growth, requiring specific media supplemented with growth factors (e.g., epidermal growth factor) to preserve the endocrine phenotype, and they have a moderate proliferation rate, with a doubling time of approximately 48-72 hours and long-term stability of characteristic functional features.
Rat parathyroid cells are the exclusive source of parathyroid hormone (PTH), which is the master regulator of systemic calcium-phosphorus homeostasis: in this hormone-producing endocrine gland, the cells detect extracellular calcium through the calcium-sensing receptor (CaSR) and secrete PTH when the extracellular calcium is low (hypocalcemia) to increase calcium reabsorption in the kidney and release from the bone. In research, this cell line is widely used to study parathyroid gland physiology, the molecular mechanisms of CaSR-mediated signaling, and pathological processes like secondary hyperparathyroidism (e.g., in chronic kidney disease). It also serves as a tool for screening drugs targeting PTH secretion or CaSR activity.
Parathyroid Cells from Patients with Secondary Hyperparathyroidism do not Respond to Changes in Extracellular Calcium
PTH regulates calcium and phosphorus homeostasis. In the pathogenesis of SHPT, a low expression of CaSR in the parathyroid gland leads to the disorder of PTH secretion. Liu's team developed an optogenetic method to inhibit PTH secretion in human hyperplastic parathyroid cells, bypassing CaSR.
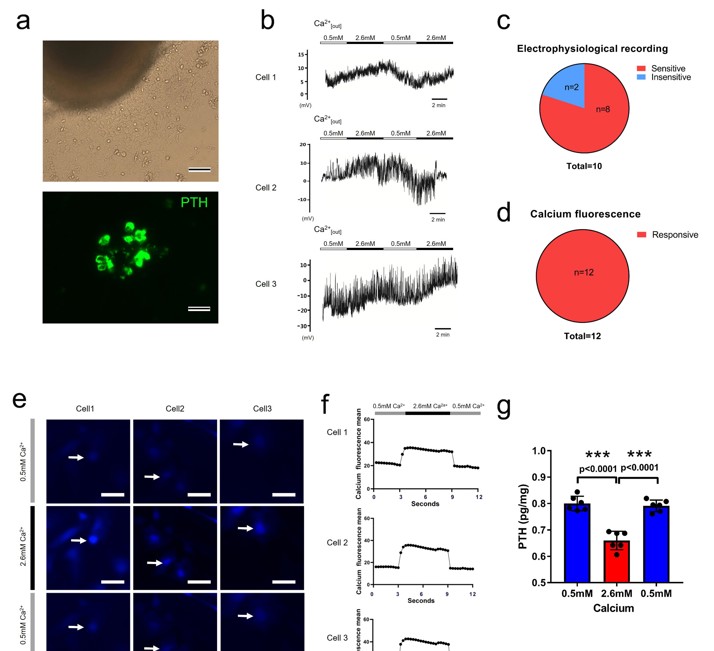
They observed that parathyroid cells from patients with SHPT do not respond to changes in extracellular calcium. To contrast this, they isolated and cultured normal rat parathyroid cells (Fig. 1a). By performing patch-clamp recordings on the normal rat parathyroid chief cell, they found that membrane potential of parathyroid chief cells in normal rats fluctuated with increasing extracellular Ca2+ from 0.5 to 2.6 mM. Among ten recorded cells (Fig. 1b), eight cells showed membrane potential fluctuation in response to high extracellular calcium (Fig. 1c). Calcium imaging and quantification data of twelve normal rat parathyroid cells showed that all the cells were sensitive to the Ca2+ increase and displayed fluorescence fluctuation during Ca2+ change (Fig. 1e, f). Moreover, the cells demonstrated a lower PTH level upon an increase in extracellular calcium (Fig. 1d). The results established a normal regulatory mechanism for PTH secretion and provided a rationale for the optogenetic therapeutic strategy (Fig. 1g).
Klotho Overexpression Suppressed the Expression of Mir-29a in Parathyroid Cells
Recently, the interaction between Klotho/fibroblast growth factor 23 (FGF-23) axis and Wnt signaling has been recognized to be responsible for chronic kidney disease (CKD)-associated comorbidities, including secondary hyperparathyroidism (SHPT). Wu's team aims to investigate the molecular mechanism of the interaction between Klotho/FGF23 axis and Wnt.
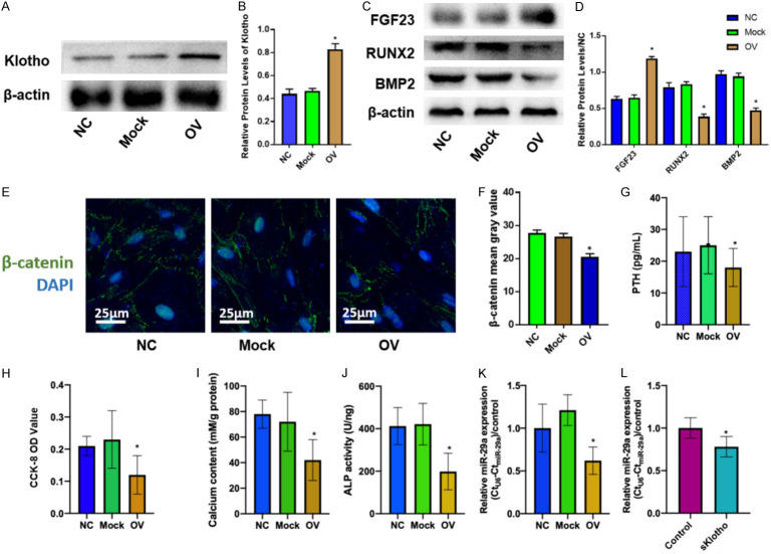
In a SHPT rat model, they observed elevated PTH, phosphorus, calcium, parathyroid calcification, and reduced Klotho. The findings above encouraged us to explore the role of Klotho/FGF23 axis in the pathogenesis of SHPT, hence lentivirus-mediated overexpression of Klotho was transfected into parathyroid cells (Fig. 2A, B). It was found that Klotho overexpression inhibited PTH secretion (Fig. 2G), calcification factors (RUNX2, BMP2; Fig. 2C, D), and β-catenin nuclear translocation (Fig. 2E, F), while increasing FGF23 (Fig. 2C, D). Klotho also suppressed parathyroid cell proliferation, calcification, and ALP activity (Fig. 2H-J), as well as miR-29a expression (Fig. 2K). Furthermore, they added Klotho protein into the media and found that miR-29a expression was suppressed in parathyroid cells (Fig. 2L). Taken together, these results suggest that the miR-29a expression is regulated by Klotho during the pathogenesis of parathyroid cells.
Ask a Question
Write your own review
- You May Also Need