- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Nucleus Pulposus Cells from Creative Bioarray are isolated from the rat intervertebral disc tissue. The method we use to isolate Rat Nucleus Pulposus Cells was developed based on a combination of established and our proprietary methods. The Rat Nucleus Pulposus Cells are characterized by immunofluorescence with antibodies specific to collagen II. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rat Nucleus Pulposus Cells are cells cultured from the nucleus pulposus of rat intervertebral discs. The nucleus pulposus is the inner core of the intervertebral disc. The nucleus pulposus consists of a gelatinous matrix with high water content and cells that are located within it. Nucleus pulposus cells operate under the distinct hypoxic physiological conditions. Nucleus pulposus cells perform various biological functions inside living organisms and in laboratory settings. Under hypoxic conditions, these cells can resist apoptosis by activating the PI3K/Akt and MEK/ERK signaling pathways. Nucleus pulposus cells can synthesize and secrete extracellular matrix, such as Type II collagen, proteoglycans, chondroitin sulfate, etc. These components can be integrated into the elastic matrix of the nucleus pulposus to maintain the structure and function of the intervertebral disc. TGF-β can induce nucleus pulposus cells to express CHSY1 (Chondroitin Sulfate Synthase 1) through the MAPK signal transduction pathway, thereby upregulating the synthesis of proteoglycans.
Nucleus pulposus cells also have an important role as a model in IVDD research. The pathophysiological process of disc degeneration can be simulated by nucleus pulposus cells in vitro, such as apoptosis, reduced synthesis of proteoglycans, etc. In gene therapy, nucleus pulposus cells can be used as targets. For example, the human TGF-β1 gene was transferred into the nucleus pulposus cells by the adenovirus vector, which can enhance the proteoglycan synthesis ability of the nucleus pulposus cells and delay the degeneration of the disc.
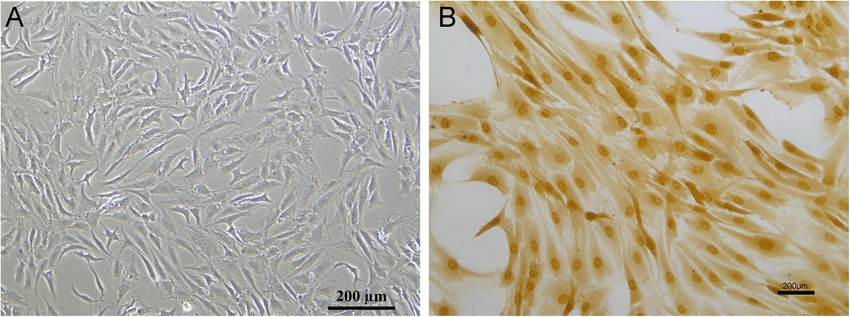 Fig. 1. Cell morphology of nucleus pulposus cells (a); immunohistochemical of collagen II (b) (Zhang H J, Ma X H, et al., 2020).
Fig. 1. Cell morphology of nucleus pulposus cells (a); immunohistochemical of collagen II (b) (Zhang H J, Ma X H, et al., 2020).
ERS Is Involved in Compression-Induced Programmed Necrosis of NP Cells
Intervertebral disc degeneration (IVDD) is a leading cause of low back pain (LBP), causing significant economic losses. The endoplasmic reticulum (ER) and mitochondria are crucial regulators of cell death, and their involvement in IVDD has been reported. However, the specific roles of ER stress (ERS) and ER-mitochondria interaction in compression-induced programmed necrosis remain unclear. Lin's team investigated the mechanisms by which ERS and ER-mitochondria interaction contribute to programmed necrosis in nucleus pulposus (NP) cells under compression.
To confirm ERS in IVDD, they compared ERS markers in normal and degenerative discs. Degenerated discs showed higher GRP78 and CHOP expression (Fig. 1a). They then tested if compression induced ERS in vitro. NP cells under compression had increased CHOP, GRP78, and p-PERK protein levels over time (Fig. 1b). Similarly, compression raised CHOP, GRP78, and PERK mRNA levels (Fig. 1c). Electron microscopy revealed ER swelling in compressed NP cells, with unclear ER-mitochondria borders at 24 and 36 hours, indicating interaction (Fig. 1d).
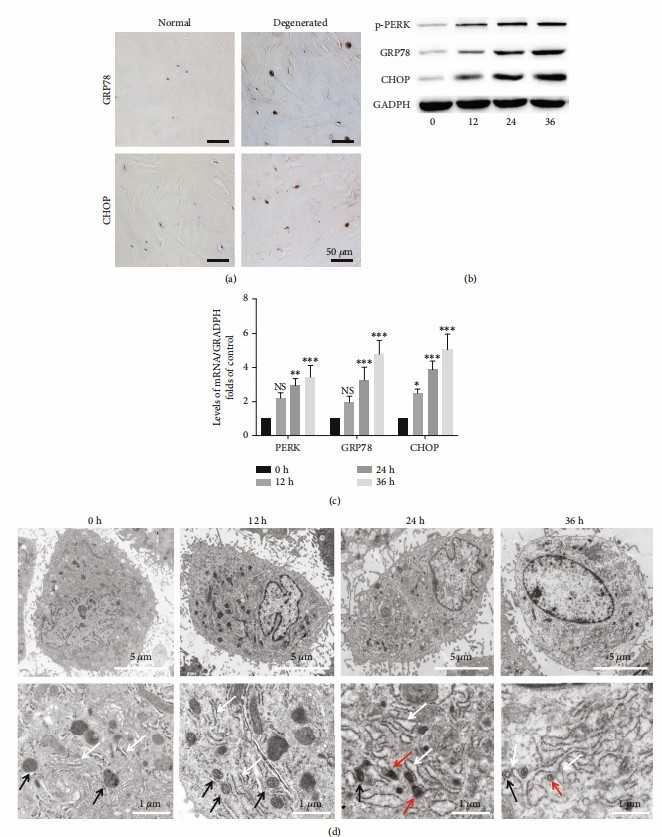 Fig. 1. ERS plays a critical role in compression-induced programmed necrosis of NP cells (Lin H, Peng Y Z, et al., 2021).
Fig. 1. ERS plays a critical role in compression-induced programmed necrosis of NP cells (Lin H, Peng Y Z, et al., 2021).
Effect of Ori on Apoptosis and ECM Degeneration in TBHP-stimulated NPCs
Restoration of SIRT1/AMPK was confirmed to prevent IVDD and damage via maintaining ER and extracellular homeostasis. orientin (Ori) has been shown to upregulate SIRT1. However, the effect of Ori in nucleus pulposus cells (NPCs) is not determined. Hence, in this study, Zhang's team aim to explore the function of Ori in IVDD pathological model.
The chemical structure of Ori is shown in Fig. 2A. Rat NPCs were treated with different concentrations (0, 10, 20, 40, and 100 μM) of Ori for 12, 24, and 48 hours. A CCK-8 assay showed no significant cytotoxic effects of Ori at any concentration or time point (Fig. 2B). Further experiments revealed that 40 μM Ori was optimal for NPCs (Fig. 2C). Ori effectively reduced apoptosis in TBHP-treated NPCs, as shown by TUNEL staining (Fig. 3A, B). RT-PCR and western blot results indicated that Ori reversed TBHP-induced changes in apoptosis-related and ECM degeneration-related biomarkers (Fig. 3C-L). Immunofluorescence showed that Ori enhanced Bcl-2 and reduced MMP13 fluorescence in TBHP-treated NPCs (Fig. 3M, N). These results suggest that Ori inhibits apoptosis and ECM degeneration in TBHP-treated NPCs. To explore the mechanisms, we focused on OS and OS-mediated ER stress.
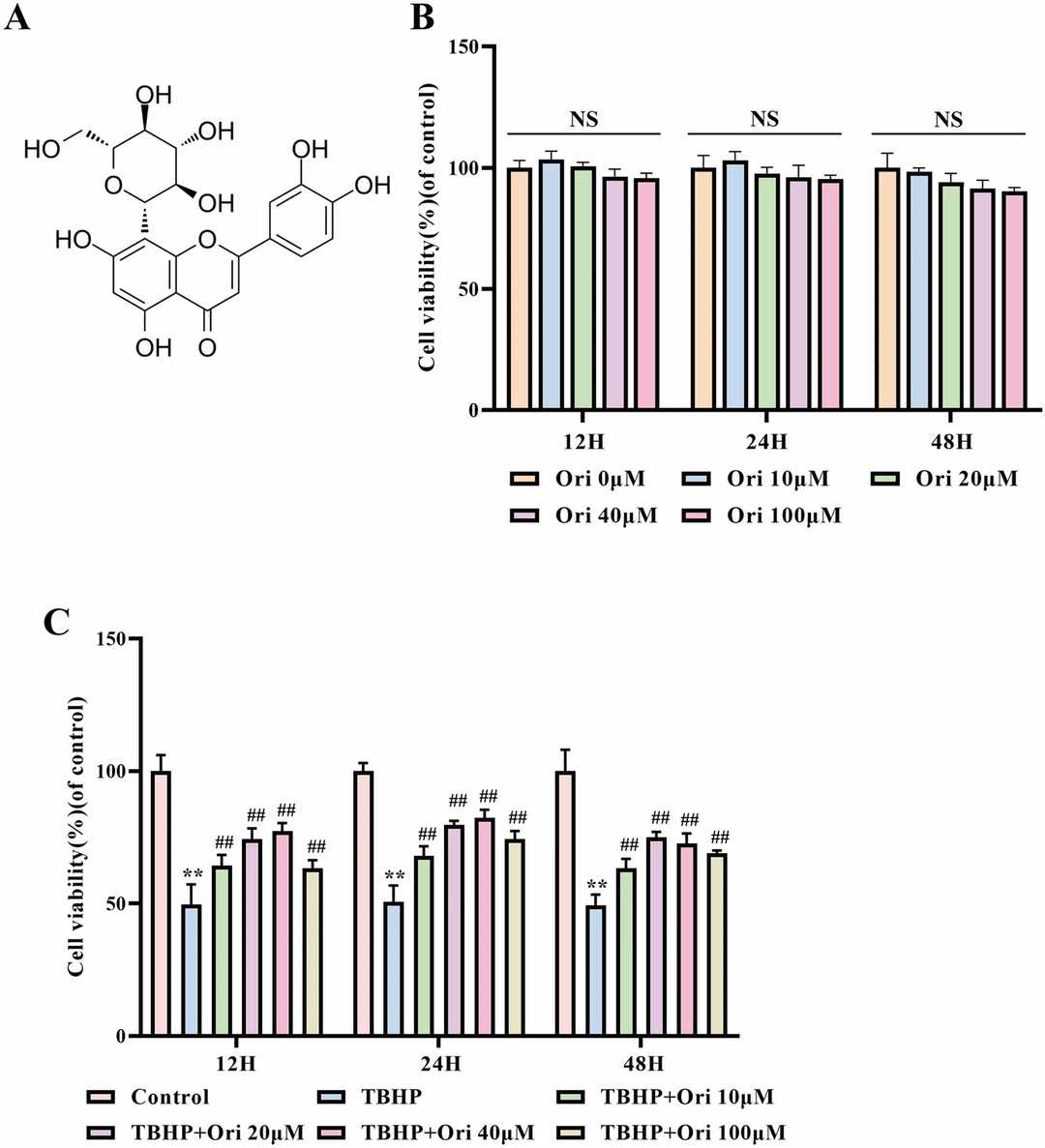 Fig. 2. The Cytotoxic Effects of Ori on NPCs (Zhang Z, Wu T, et al., 2022).
Fig. 2. The Cytotoxic Effects of Ori on NPCs (Zhang Z, Wu T, et al., 2022).
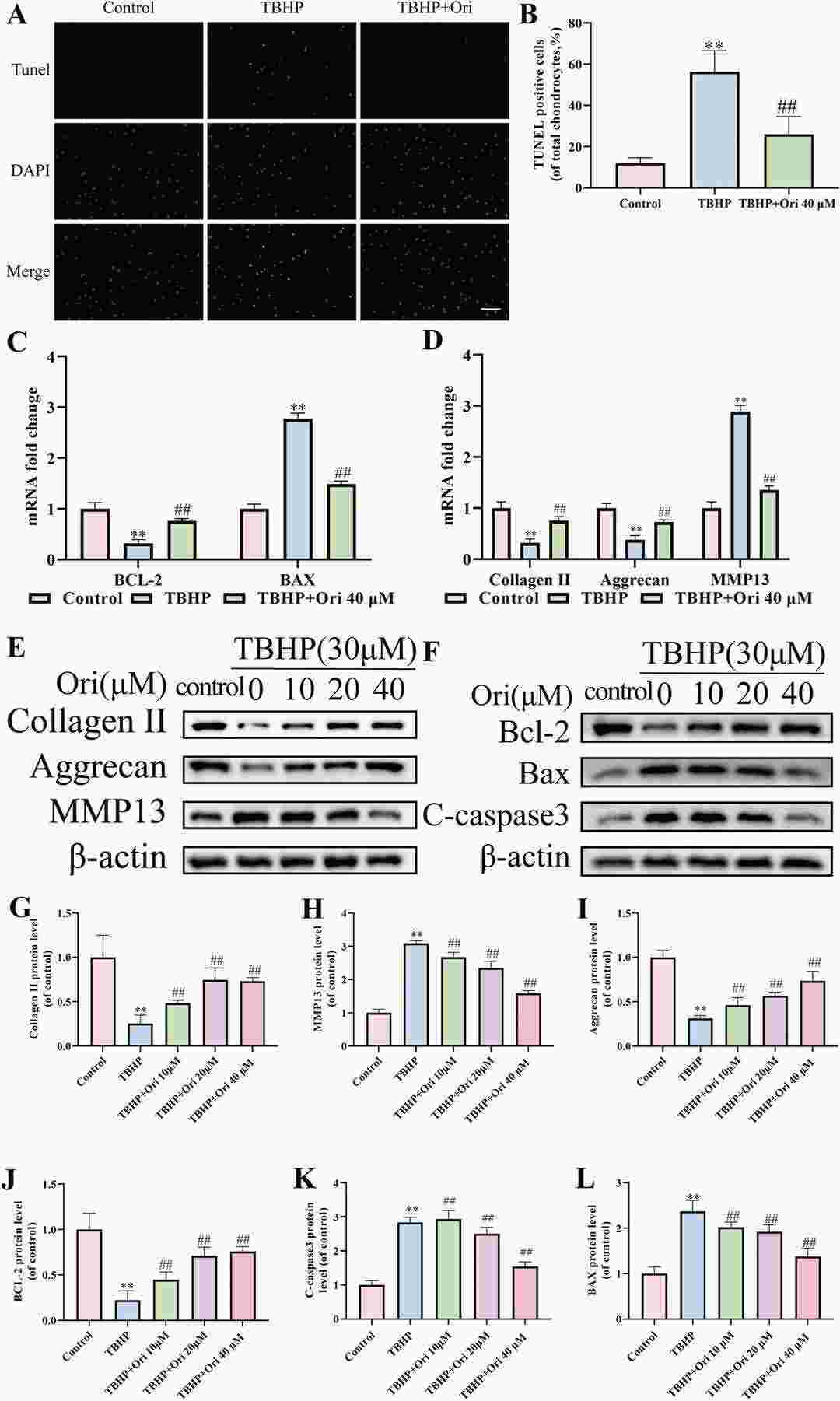 Fig. 3. The Effect of Ori on Apoptosis and ECM degeneration in TBHP-Stimulated NPCs (Zhang Z, Wu T, et al., 2022).
Fig. 3. The Effect of Ori on Apoptosis and ECM degeneration in TBHP-Stimulated NPCs (Zhang Z, Wu T, et al., 2022).
Ask a Question
Write your own review