Rat Cavernous Endothelial Cells
Cat.No.: CSC-C5112S
Species: Rat
Source: Penis
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Cavernous Endothelial Cells from Creative Bioarray are isolated from the rat penis tissue. The method we use to isolate Rat Cavernous Endothelial Cells was developed based on a combination of established and our proprietary methods. The Rat Cavernous Endothelial Cells are characterized by immunofluorescence with antibodies specific to von Willebrand factor (vWF). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rat Cavernous Endothelial Cells (RCECs) are primary endothelial cells isolated from the sinusoidal spaces within the rat corpus cavernosum, a highly vascularized erectile tissue essential for penile hemodynamics. Characteristically, RCECs grown in vitro display a cobblestone morphology and express typical endothelial markers (e.g., CD31, VE-cadherin). They are integral in mediating erectile function through nitric oxide (NO)-dependent vasodilation and maintaining vascular homeostasis.
RCECs are highly sensitive to metabolic stressors such as high glucose and free fatty acids, leading to endothelial dysfunction and a mesenchymal phenotype shift (EndMT), characterized by the upregulation of α-SMA and vimentin. This pathological transition contributes to fibrosis and erectile dysfunction (ED), especially in diabetic models. While primary RCECs have limited proliferative potential, immortalized lines (e.g., v-myc-transfected vRECs) have been developed for extended studies.
RCECs are widely used in erectile dysfunction (ED) research and serve as a key cellular model for exploring molecular mechanisms (e.g., YAP signaling in fibrosis), drug screening (e.g., PDE5 inhibitors), and vascular remodeling.
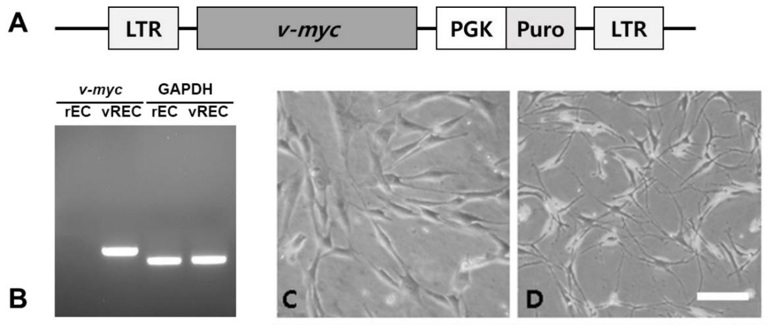
Immortalization of Penile Cavernous Endothelial Cells
The leading cause of erectile dysfunction (ED) is injury to penile cavernous endothelial cells (ECs). Primary ECs from mouse showed limited growth capacity, however the ease of harvest made it a useful model to study cavernous endothelial dysfunction. In this study, immortalized rat penile cavernous ECs (rECs) was established and its application in ED repair was investigated in a rat cavernous nerve injury (CNI) model.
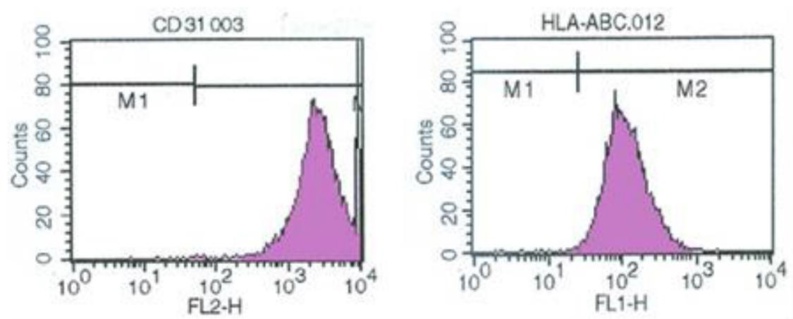
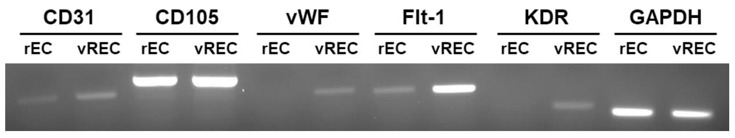
rECs were isolated by collagenase digestion and cultured. An amphotropic retroviral vector encoding v-myc oncogene was used to immortalize the cells (vRECs). RT-PCR was performed to confirm v-myc expression in immortalized rECs (Fig. 1B). Confluence was reached after 7-10 days of growth and a "cobblestone" morphology was observed. The cultures showed no spindle cell contamination (Fig. 1C). CD31 sorting was performed and 95% purity was achieved for rECs. After immortalization, vRECs (Fig. 1D) were able to grow past 70 population doublings without entering crisis. Flow cytometry was performed to isolate the vRECs, which were found to be mostly expressing CD31 (Fig. 2). Endothelial cell specific markers (CD31, CD105, vWF, Flt-1, KDR, GAPDH) were expressed highly in the vRECs (Fig. 3).
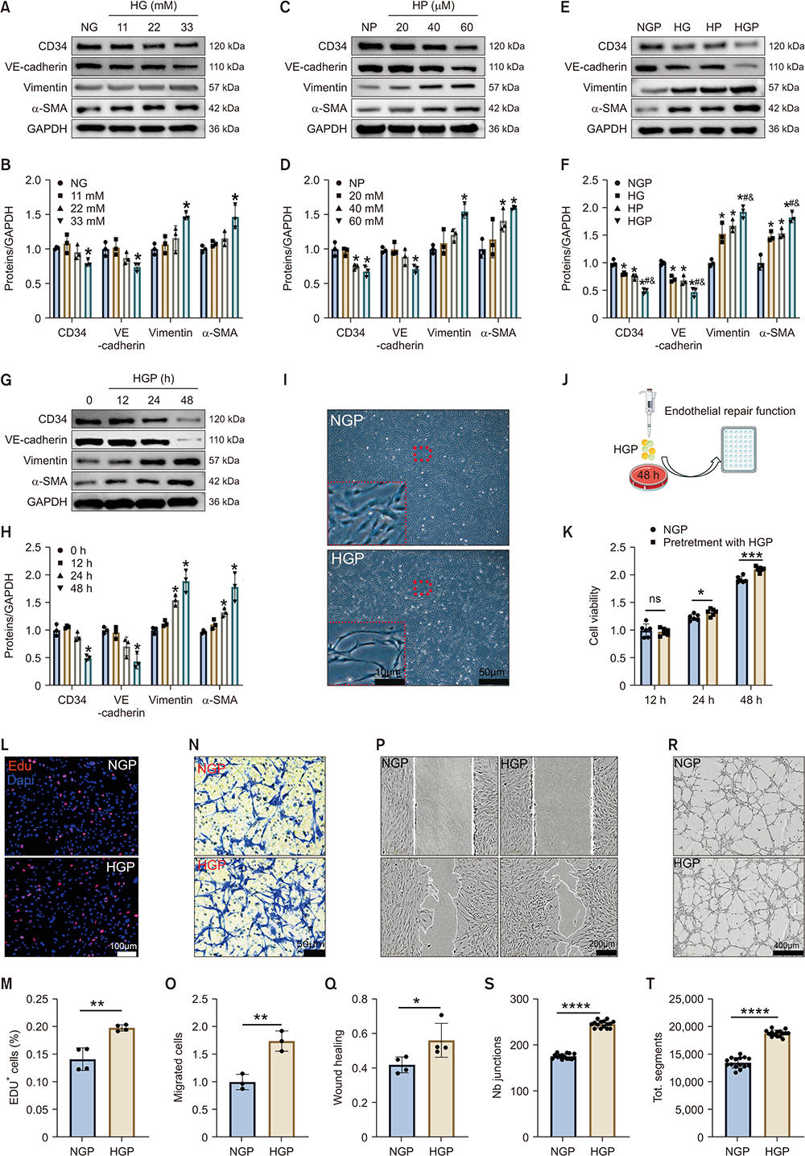
HGP Promotes EndMT in RCECs in vitro
Endothelial-mesenchymal transition (EndMT), a cellular mechanism of fibrosis, trans-differentiates endothelial cells into matrix-secreting fibroblast-like cells. YAP is a crucial EndMT regulator and its upregulation has been observed in DMED. Xiao's team explored if YAP inhibition in cavernous endothelial cells could block endMT and restore erectile function in diabetic rats.
Rat cavernous endothelial cells (RCECs) were incubated with HG and PA to establish a diabetic type 2 model. HG or HP independently decreased the expression of endothelial markers (CD34, VE-cadherin) and increased mesenchymal markers (α-SMA, vimentin) (Fig. 4A-D). HGP treatment showed a time-dependent trend, and the effects were exacerbated in the combined HG and HP condition (Fig. 4E-H). HG alone showed no significant effect on RCEC viability at 48h, whereas, HP treatment exhibited obvious cytotoxicity. HGP time-dependently reduced RCEC activity, and trans-differentiated RCECs from short-spindle to elongated morphology (Fig. 4I). After 48h of HGP treatment only mesenchymal trait-exhibiting RCECs remained viable. The functional analysis indicated that HGP increased interstitial RCEC activity (Fig. 4K), proliferation (Fig. 4L-M), migration (Fig. 4N-Q), and tubule formation (Fig. 4R-T). These results indicated that the EndMT induced by HGP may play a compensatory role in tissue repair after injury.
Ask a Question
Write your own review
- You May Also Need