Rat Aortic Valve Interstitial Cells
Cat.No.: CSC-C5090S
Species: Rat
Source: Heart
Cell Type: Interstitial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Aortic Valve Interstitial Cells (rAoVICs) from Creative Bioarray are isolated from the rat aortic tissue. The method we use to isolate rAoVICs was developed based on a combination of established and our proprietary methods. The rAoVICs are characterized by immunofluorescence with antibodies specific to α-SMA or vimentin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Rat aortic valve interstitial cells (AVICs) isolated from rat aortic valves are interstitial cells located mainly within the fibrosa and spongiosa layers and they maintain the structural integrity and functional operation of the valve. The pathophysiology of the aortic valve depends significantly on the functions of AVICs. They are not only involved in maintaining the structural integrity of the valve but also regulate its mechanical properties by secreting extracellular matrix (ECM) components such as collagen and elastin. Furthermore, these cells exhibit high phenotypic plasticity, capable of differentiating into fibroblasts and myofibroblasts, thus playing significant roles in valve development, disease, and repair processes.
AVICs serve as crucial models to understand the processes leading to calcific aortic valve disease (CAVD). Research findings reveal that high glucose levels cause AVIC calcification through elevated osteocalcin (ALP) expression and bone morphogenetic protein (BMP) synthesis which helps understand valve calcification in diabetes patients. Additionally, studies revealed that osteogenic differentiation of AVICs can be blocked through specific signaling pathway inhibitors including NF-κB, ERK, and AKT which presents potential therapeutic targets.
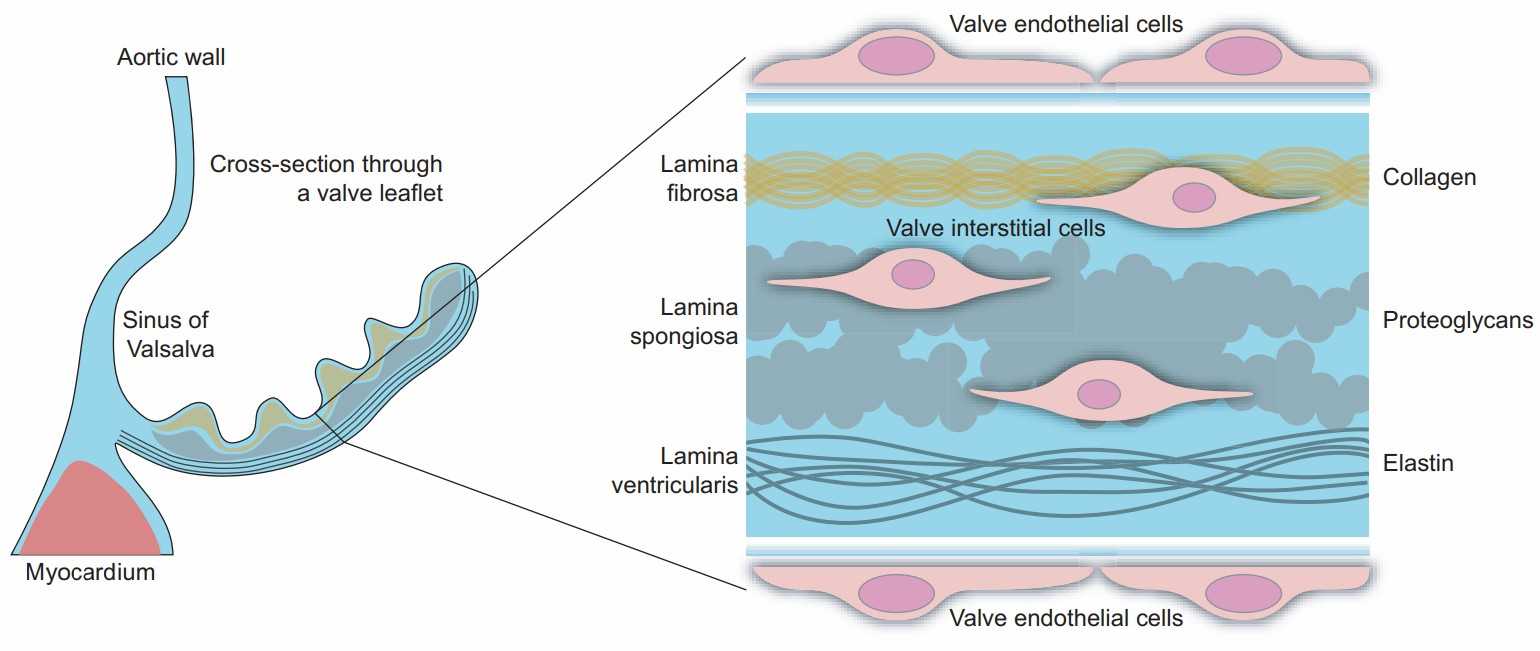 Fig. 1. Simplified structure of the human aortic valve leaflet. The blowup on the right shows the trilayered organization of the extracellular matrix and the localization of the aortic valve endothelial cells (shortened throughout to VECs) and interstitial cells (VICs) (Rutkovskiy A, Malashicheva A, et al., 2017).
Fig. 1. Simplified structure of the human aortic valve leaflet. The blowup on the right shows the trilayered organization of the extracellular matrix and the localization of the aortic valve endothelial cells (shortened throughout to VECs) and interstitial cells (VICs) (Rutkovskiy A, Malashicheva A, et al., 2017).
Glycosaminoglycans Affect Endothelial to Mesenchymal Transformation, Proliferation, and Calcification in a 3D Model of Aortic Valve Disease
CAVD involves multifaceted processes in the valve microenvironment, resulting in matrix reorganization, calcification, and functional impairment. Current valve replacements do not biologically mimic native valves.
In this study, a 3D collagen hydrogel co-culture model of the aortic valve fibrosa was created to study the role of EndMT-derived activated valvular interstitial cell behavior in CAVD progression. Porcine aortic valve interstitial cells (PAVIC) and porcine aortic valve endothelial cells (PAVEC) were cultured within collagen I hydrogels containing the GAGs chondroitin sulfate (CS) or hyaluronic acid (HA). After 14 days in culture, samples were tested for early ALP activity and late-stage calcification using ARS assays. All conditions showed ALP activity below 0.11 mg p-nitrophenol/mg protein, with insignificant differences between GAG conditions and the 1.5 mg/mL collagen-only controls. The collagen stiffness control at 2.2 mg/mL was notably higher than both GAG conditions, indicating stiffness plays a greater role in early disease stages than GAG presence (Fig. 1A). Alizarin Red S staining identified calcific nodule formation in hydrogels, with stained hydrogels imaged (Fig. 1D–G) and analyzed via ImageJ. Plate reader quantification showed that increased stiffness (2.2 mg/mL collagen) promoted calcification, with 20 mg/mL CS significantly enhancing matrix mineralization, while HA did not (Fig. 1B). Stain quantification matched the stained area quantification in ARS images (Fig. 1C).
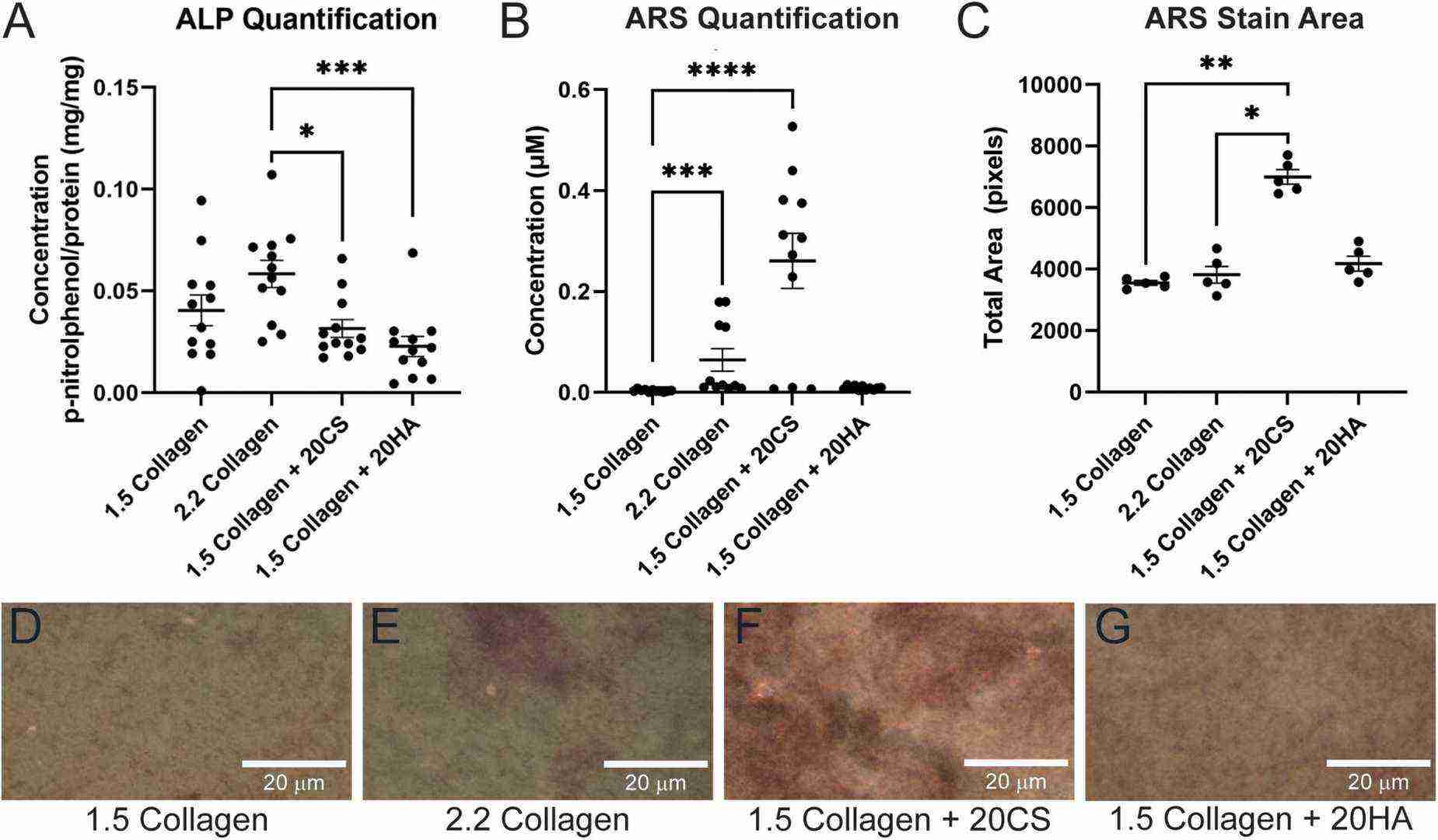 Fig. 1. Chondroitin sulfate promotes calcific nodule formation and glycosaminoglycans (GAGs) do not produce increased alkaline phosphatase (ALP) activity (Bramsen J A, Alber B R, et al., 2022).
Fig. 1. Chondroitin sulfate promotes calcific nodule formation and glycosaminoglycans (GAGs) do not produce increased alkaline phosphatase (ALP) activity (Bramsen J A, Alber B R, et al., 2022).
Krox20 Regulates Endothelial Nitric Oxide Signaling in Aortic Valve Development and Disease
BAV, a result of abnormal leaflet fusion during development, involves complex interactions among neural crest and endocardial derivatives. Nos3's expression is critical for valve development due to its sensitivity to hemodynamic signals, and its dysregulation is linked to BAV. Despite recent advances, the etiology of BAV is poorly understood. Odelin et al. have recently shown that Krox20 is expressed in endothelial and cardiac neural crest derivatives that normally contribute to aortic valve development and that lack of Krox20 in these cells leads to aortic valve defects including partially penetrant BAV formation.
Odelin et al. used compound heterozygous Krox20+/?;Nos3+/? mice to assess BAV malformations and conducted in vivo and in vitro analyses to demonstrate Krox20's regulatory effect on Nos3. Laforest et al. identified conserved GATA binding sites in the murine ?1.5-kb Nos3 promoter and showed that Gata5 enhances Nos3 promoter activity via these sites. Based on this, they examined the Nos3 promoter for conserved Krox20 binding sites. Bioinformatics analysis revealed two conserved Krox20-binding sites (K1 and K2) in the Nos3 proximal promoter (Fig. 2A). EMSA experiments showed that Krox20 binds to these sites with higher affinity for K2 (Fig. 2B). Luciferase assays indicated that Krox20 overexpression in Cos7 cells increased transcriptional activity of the ?1500 bp region up to 2.5-fold, but not the ?265 bp promoter (Fig. 2C). Mutation of the K2 site abolished Krox20-mediated activation of the ?1500 bp promoter, confirming its importance. ChIP experiments on E13.5 OFT and ventricle tissues showed that Krox20 binds specifically to the A region of the Nos3 promoter in OFT (Fig. 2D). Additionally, Krox20 transfection in rat aortic valve interstitial cells (AVICs) activated Nos3 expression (Fig. 2E). These findings identify Krox20 as a Nos3 promoter activator and suggest that reduced Nos3 expression may contribute to BAVs in Krox20 mutant mice.
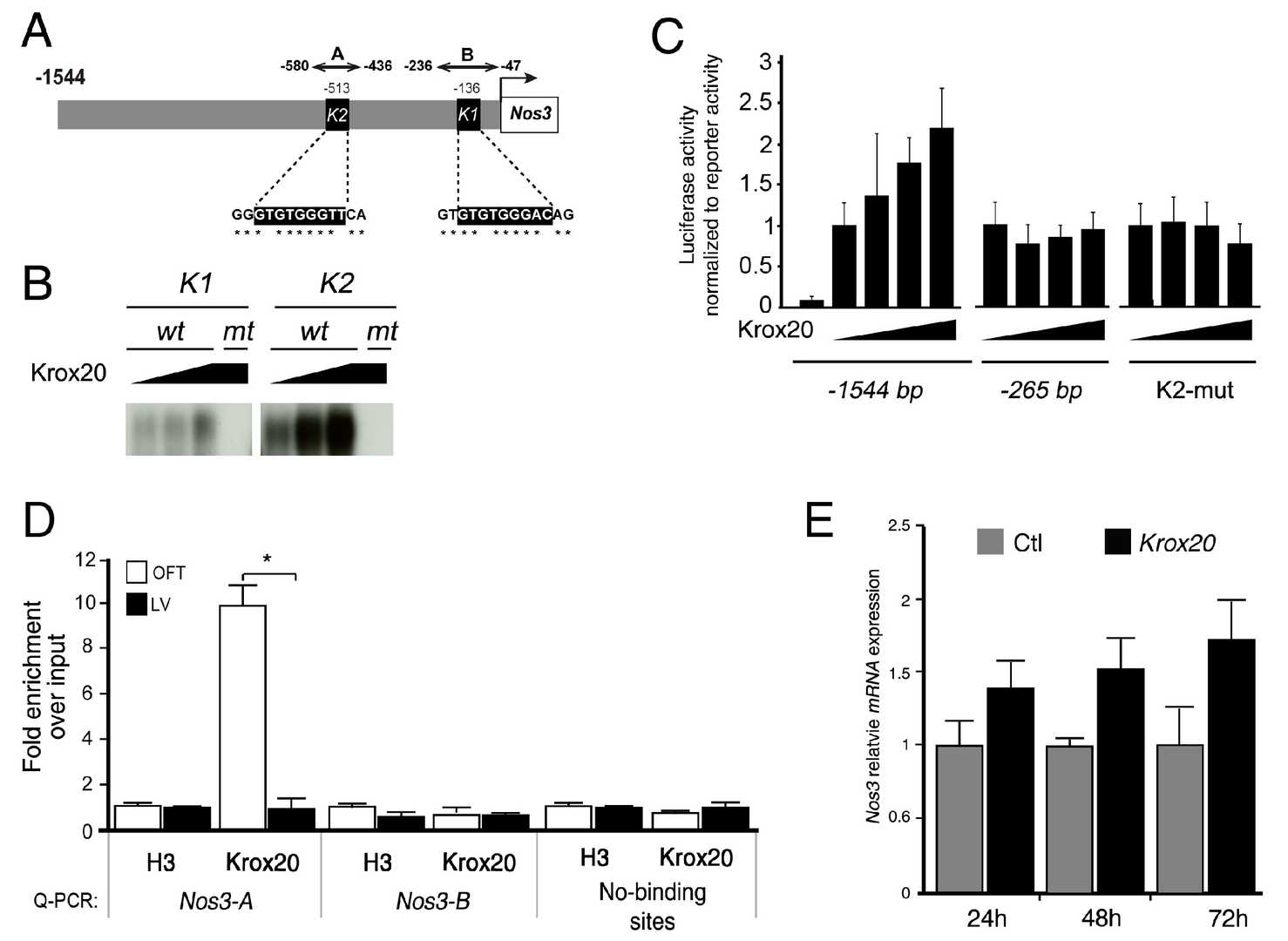 Fig. 2. Krox20 promotes the transcriptional activity of the Nos3 proximal promoter (Odelin G, Faure E, et al., 2019).
Fig. 2. Krox20 promotes the transcriptional activity of the Nos3 proximal promoter (Odelin G, Faure E, et al., 2019).
Ask a Question
Write your own review