- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Adrenocortical Cells from Creative Bioarray are isolated from the rat adrenal tissue. The method we use to isolate Rat Adrenocortical Cells was developed based on a combination of established and our proprietary methods. The Rat Adrenocortical Cells are characterized by immunofluorescence with antibodies specific to 3β-HSD. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Adrenal cortical cells originate primarily from the cortex of the rat adrenal gland which includes cells from the zona glomerulosa, zona fasciculata, and zona reticularis. These cells form an organized structure which consists of three distinct layers. Microscopic examination reveals unique cell morphologies across different regions. The zona glomerulosa cells show cube or columnar shapes with dense cytoplasm packed with rough endoplasmic reticulum and mitochondria and they possess small nuclei that display dark staining. Zona fasciculata cells develop mature forms with higher cholesterol side-chain cleavage enzyme (P450scc) expression in the cytoplasm while keeping their nuclei small and lighter in staining appearance. Zona reticularis cell cytoplasm shows many lipid droplets and displays nuclei which stain lightly yet appear enlarged.
These cells specialize in hormone production. Aldosterone secretion from zona glomerulosa cells controls sodium balance in the body. Glucocorticoids produced by zona fasciculata cells help manage carbohydrate metabolism while regulating immune functions and responding to stress. Androgen secretion by zona reticularis cells supports gonadal development and sexual function. Hormone synthesis triggers multiple signaling pathways that involve cAMP, PKA, MAPK, ERK, and PI3K/Akt.
Researchers use rat adrenal cortical cells as an ideal model to examine the steroid hormone synthesis pathways. With these cellular systems researchers can examine cellular responses to hormones such as ACTH, cAMP and fatty acids to understand the molecular mechanisms behind hormone synthesis. At the same time, these cells demonstrate significant differentiation capabilities while functioning as critical instruments for investigating the processes of cell differentiation and tissue regeneration. Cells transition from the zona glomerulosa to the zona fasciculata or zona reticularis during in vitro culture conditions.
Impact of Classic Adrenal Secretagogues on mRNA Levels of Urotensin II and Its Receptor in Adrenal Gland of Rats
Adrenal hormones regulate key physiological processes, with ACTH, AngII, and K⁺ ions being major controllers. Urotensin II (Uts2) and its receptor (Uts2r) are expressed in the adrenal glands and involved in tumor development and steroidogenesis, but their regulation by adrenal secretagogues is unknown.
Jopek et al. investigated the impact of ACTH, AngII, and K⁺ on Uts2 and Uts2r mRNA levels in rat adrenal glands. In vitro experiments were conducted on freshly isolated and cultured rat adrenocortical cells. In the primary culture of rat adrenocortical cells, ACTH significantly increased the Uts2 transcript level. Treatment with K+ and AngII did not affect the expression of the studied mRNAs. Furthermore, ACTH, AngII, or K+ had no effect on the Uts2r mRNA level in freshly isolated rat adrenocortical cells. The Uts2r transcript level in primary cultured cells was undetectable by qPCR and thus excluded from analysis. Next, they determined the urotensin II concentration in the incubation medium of freshly isolated adrenocortical cells. They also quantified corticosterone and aldosterone levels in the medium as a positive control of adrenocortical cell functions. As expected, ACTH-induced secretion of corticosterone was observed in both ZG and ZF/R, and aldosterone secretion was elevated in ZG cells stimulated with ACTH, AngII, and K+. Surprisingly, all applied secretagogues downregulated urotensin II secretion in ZG cells (Fig. 1).
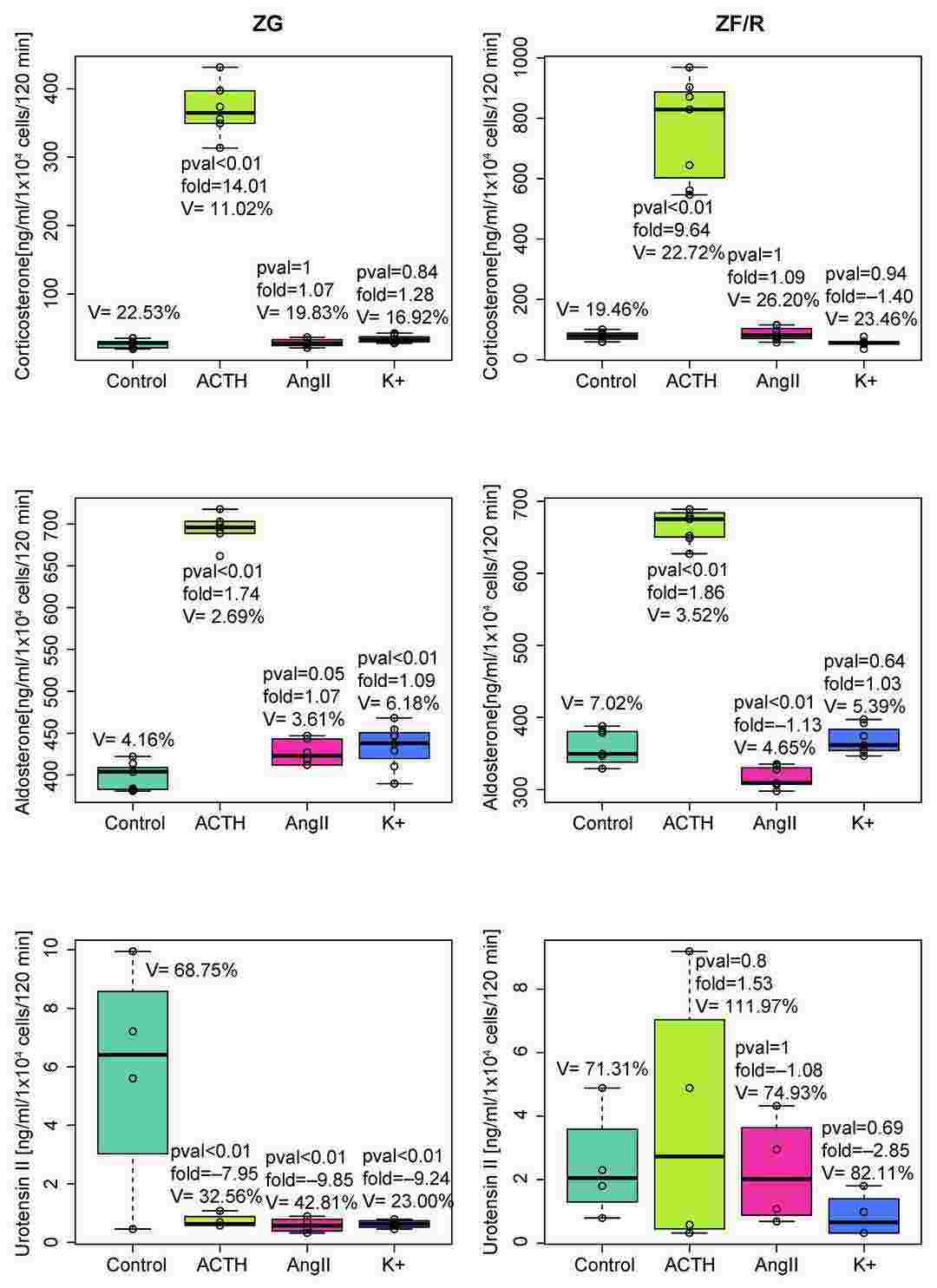 Fig. 1. Urotensin II, aldosterone, and corticosterone outputs into incubation medium by freshly isolated ZG or ZF/R rat adrenocortical cells exposed for 120 min to ACTH, AngII, or K+ (Jopek K, Tyczewska M, et al., 2023).
Fig. 1. Urotensin II, aldosterone, and corticosterone outputs into incubation medium by freshly isolated ZG or ZF/R rat adrenocortical cells exposed for 120 min to ACTH, AngII, or K+ (Jopek K, Tyczewska M, et al., 2023).
Nicotinamide Phosphoribosyltransferase and the Hypothalamic-Pituitary-Adrenal Axis of the Rat
Nicotinamide phosphoribosyltransferase (Nampt), also known as visfatin, is a key enzyme in the NAD salvage pathway. It exists in intracellular (iNampt) and extracellular (eNampt) forms. iNampt is crucial for NAD synthesis, while eNampt acts as a cytokine influencing various pathways. Despite its diverse roles, the involvement of Nampt in the hypothalamic-pituitary-adrenal (HPA) axis remains unclear.
Adrenal compartment isolation and freshly isolated rat adrenocortical cells for in vitro experiments. In freshly isolated ZF/R cells, 0.1 nM eNampt increased corticosterone output, unlike 10 nM (Fig. 2A). eNampt did not affect ACTH-stimulated corticosterone secretion in freshly isolated cells (Fig. 2B). In primary cell cultures, 1 and 100 nM eNampt for 24 hours on day 4 did not change basal aldosterone and corticosterone secretion; ACTH response remained (Fig. 3A and B). Increased Nampt gene expression was observed in eNampt-treated cells, but not with ACTH (Fig. 3C). The study also used FK866, a specific iNampt inhibitor, showing reduced basal aldosterone output without eNampt alteration (Fig. 4A). FK866 eliminated ACTH's stimulation on aldosterone output (Fig. 4B) and similarly on corticosterone output (Fig. 4C and D).
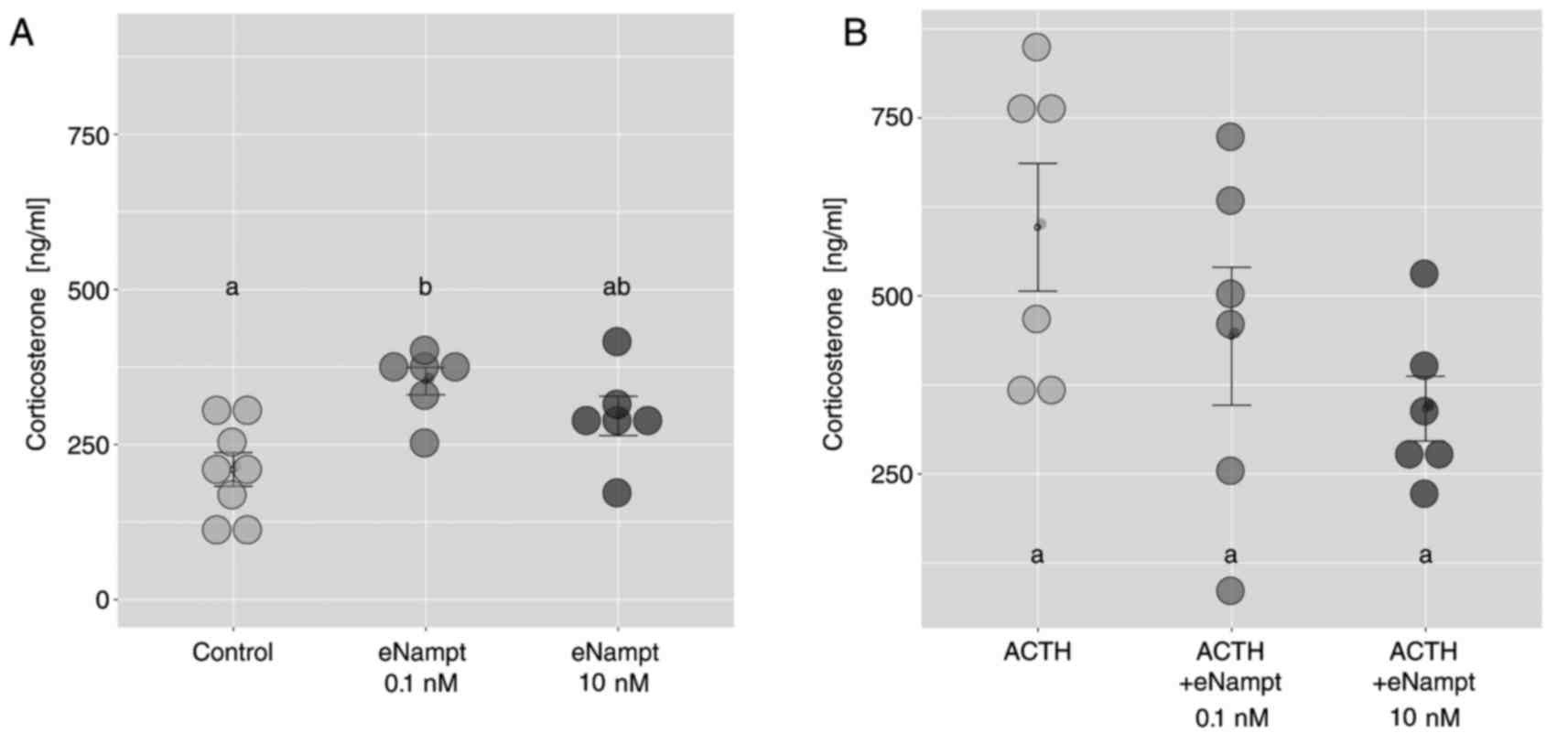 Fig. 2. Effects of eNampt on basal and ACTH-stimulated corticosterone output by freshly isolated zona fasciculata/reticularis adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
Fig. 2. Effects of eNampt on basal and ACTH-stimulated corticosterone output by freshly isolated zona fasciculata/reticularis adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
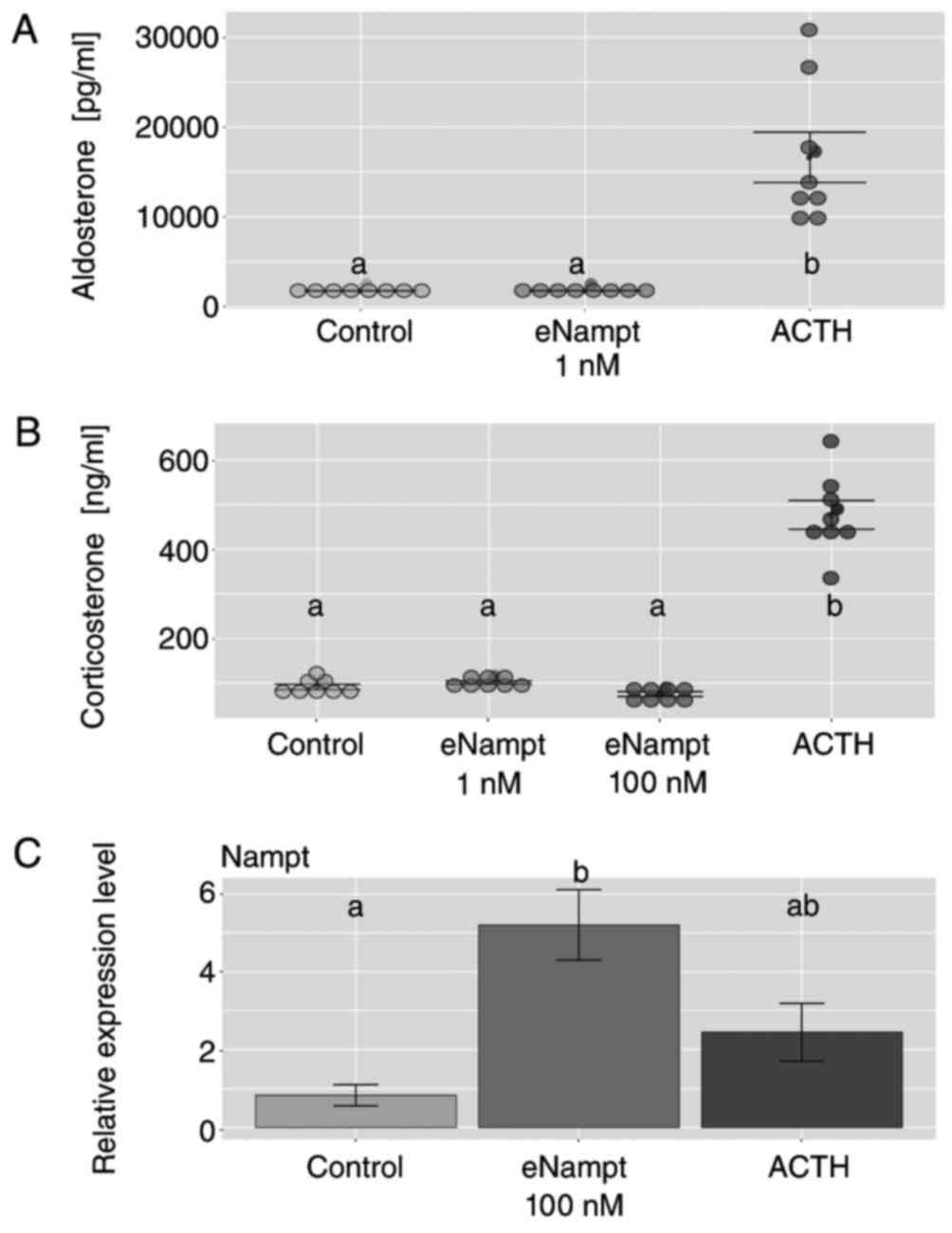 Fig. 3. Effects of eNampt on aldosterone and corticosterone output in primary cultures of rat adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
Fig. 3. Effects of eNampt on aldosterone and corticosterone output in primary cultures of rat adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
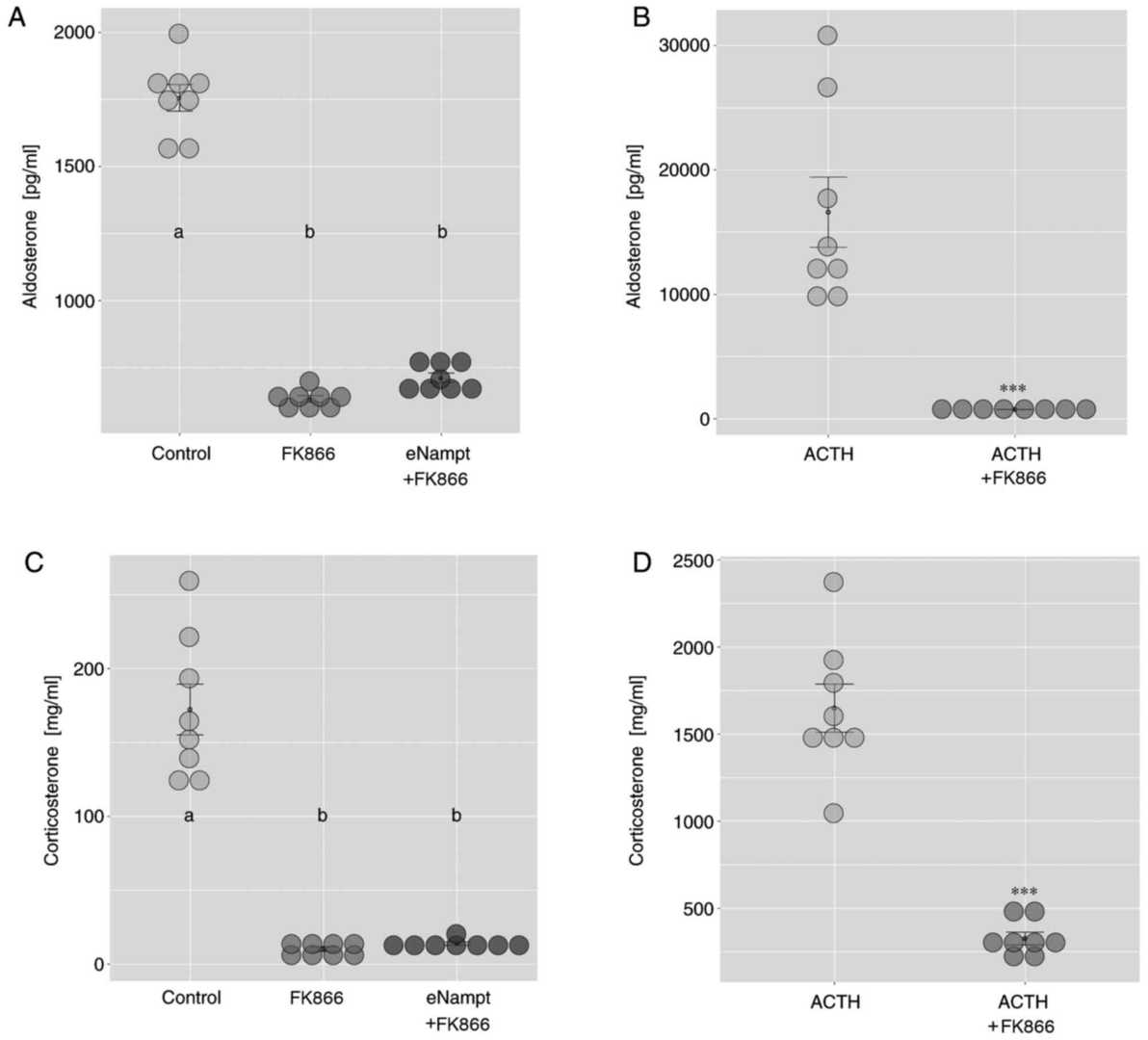 Fig. 4. Effects of FK866 on basal and ACTH-stimulated aldosterone and corticosterone output in primary cultures of rat adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
Fig. 4. Effects of FK866 on basal and ACTH-stimulated aldosterone and corticosterone output in primary cultures of rat adrenocortical cells (Crlichowski P, Jopek K, et al., 2018).
Ask a Question
Write your own review
- You May Also Need