
Immortalized Human Skeletal Muscle Cells
Cat.No.: CSC-I9094L
Species: Homo sapiens
Source: Skeletal Muscle
Morphology: Multipolar
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Immortalized Human Skeletal Muscle Cells are derived from human myoblasts that acquire unlimited proliferation capacity through immortalization. These cells are derived from adult skeletal muscle, such as the biceps femoris, which are involved in movement and posture. In culture, these cells typically grow in a monolayer, and when differentiated, can form multinucleated myotubes and exhibit typical muscle fiber morphology.
These cells primarily generate force through contraction, which results in movement. Skeletal muscle cells play a crucial role in energy metabolism, participate in immune regulation through cytokine secretion, and are used as a model for studying neuromuscular diseases such as Duchenne muscular dystrophy (DMD) and amyotrophic lateral sclerosis (ALS). Additionally, the cell lines are used for drug screening and toxicity testing, especially in relation to muscle function and neuromuscular junction activity. They are also important in the field of regenerative medicine and tissue engineering, where they are used to construct engineered muscle bundles for regeneration and repair.
Simplified In Vitro Engineering of Neuromuscular Junctions between Rat Embryonic Motoneurons and Immortalized Human Skeletal Muscle Cells
Neuromuscular junctions (NMJs) are critical structures where motoneuron terminals connect with skeletal muscle fibers, facilitating muscle contraction and movement through the release of acetylcholine. Despite extensive research, in vivo investigations of NMJs face limitations.
Saini et al. have developed an easy and reproducible serum-free coculture of immortalized human skeletal muscle cells (SKMCs) and embryonic rat spinal cord explants that induces a rapid formation of functional NMJs. The first spontaneous contractions of myotubes were observed 3 days after co-culturing SkMCs with rat embryo spinal cord explants. This constituted a milestone in the early development of a nerve–muscle coculture system. They did observe contractions after 72 hours, but these were predominantly individual and arrhythmic, and mainly occurred among myotubes in the immediate proximity of the explant. The number and frequency of contracting myotubes increased gradually, such that from day 7 onwards, networks of myotubes contracted rhythmically, as a single motor unit. Detached contracting myotubes were characteristically three-dimensional in appearance, in contrast to aneurally cultured myotubes, which remained flat and failed to contract. Together, these results establish that the NMJs we have observed are functional, although multiple innervations were still present in a significant proportion (83.4±12.6%, n=20) of innervated myotubes. Mature myotubes in vivo have transverse triads that are made up of T-tubules and terminal cisternae. The T-tubules were observed by staining for the dihydropyridine receptor (DHPR), whereas the terminal cisternae were observed by staining for the ryanodine receptor (RyR). Well-defined triads were observed in around 32% of cocultured myotubes after 14 days (Fig. 1A), in contrast to none of the aneurally cultured SkMCs (Fig. 1B, C).
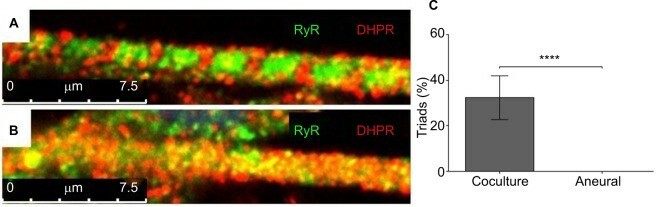 Fig. 1. Characterization of myotubes at day 14 (Saini J, Faroni A, et al.,2019).
Fig. 1. Characterization of myotubes at day 14 (Saini J, Faroni A, et al.,2019).
Cross-Talk between Motor Neurons and Myotubes Via Endogenously Secreted Neural and Muscular Growth Factors
Studies of neuromuscular junctions (NMJs) provide important insights into the basic mechanisms of neuromuscular pathology and are needed for pre-clinical testing of new therapies to treat NM dysfunction. The NMJ is also highly influenced by the surrounding microenvironment. Saini et al. took advantage of a co-culture platform of immortalized human skeletal muscle cells with rat embryonic motor neurons to investigate the endogenous secretion of growth factors.
To analyze cross-talk between muscle and motor neurons, they used the co-culture NMJ model developed in our lab (Fig. 2a). At day 7 of the co-culture platform, cholinergic motor neurons from the rat-embryo spinal-cord explants sprouted and branched to form several neuromuscular innervation sites (Fig. 2a). At the same time, the immortalized human myoblasts differentiated into striated myotubes with peripherally located nuclei, and expressed acetylcholine receptors, as demonstrated by the α-BTX stain as red clusters, thus confirming NMJ formation (Fig. 2a). In the absence of neuronal inputs, i.e. aneural cultured myoblast, the cells did not show such a high degree of differentiation, as they lacked cross-striation and peripherally located nuclei (Fig. 2b).
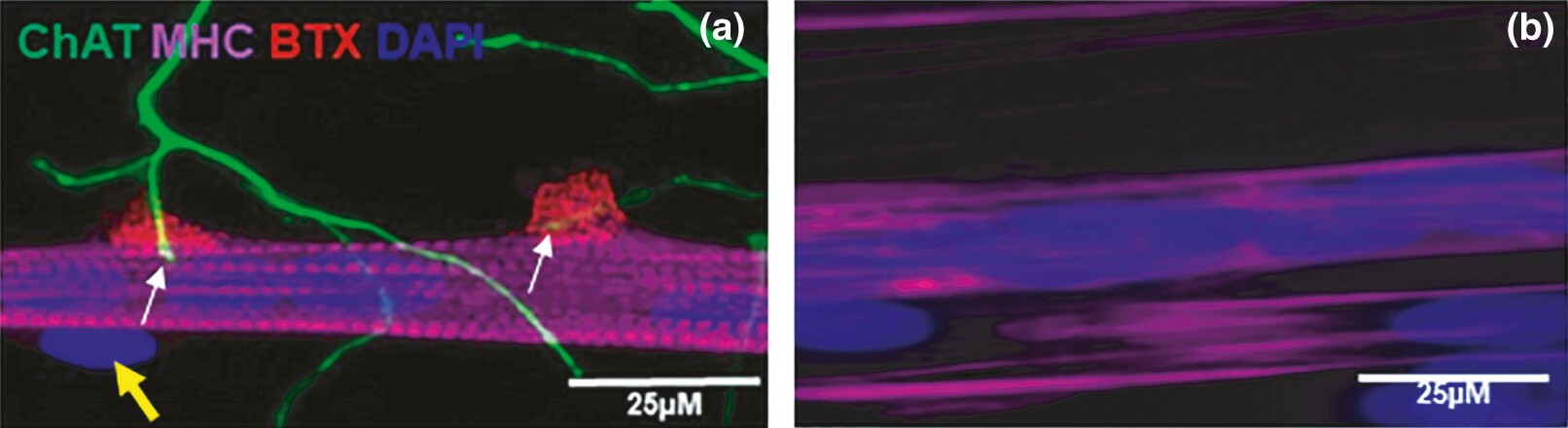 Fig. 2. Image of neuromuscular junctions (NMJ) in the co-culture of immortalized human skeletal muscle cells with rat-embryo spinal-cord explants (a) and aneurally cultured immortalized human skeletal muscle cells (b) at day 7 (Saini J, Faroni A, et al., 2021).
Fig. 2. Image of neuromuscular junctions (NMJ) in the co-culture of immortalized human skeletal muscle cells with rat-embryo spinal-cord explants (a) and aneurally cultured immortalized human skeletal muscle cells (b) at day 7 (Saini J, Faroni A, et al., 2021).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
