
Immortalized Human Olfactory Ensheathing Glia-Bmi1/hTERT
Cat.No.: CSC-I9086L
Species: Homo sapiens
Source: Oflactory Bulbs
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
The "immortalized human olfactory ensheathing glia-Bmi1/hTERT" cell line originates from human olfactory bulb tissue and achieves immortality via lentivirus-mediated transfection of Bmi1 and hTERT genes. The olfactory bulb which sits at the nasal cavity base plays a crucial role in the olfactory system through its function of receiving external odorants and transmitting these signals to the brain's olfactory cortex. Olfactory ensheathing glial cells support central nervous system repair through their role in neuronal axon regeneration. Recent scientific research reveals that these cells support axonal regeneration in retinal ganglion cells and display significant neuroregenerative properties during spinal cord injury research. Furthermore, multiple neurotrophic factors and extracellular matrix components like fibronectin and laminin are produced by these cells for neural regeneration support.
Their capacity to repair central nervous system damage makes olfactory ensheathing glial cells essential in regenerative therapy for spinal cord injuries, brain damage and retinal disorders. For instance, research indicates that these cells promote axonal repair in injured neurons from spinal cord injury models which results in recovered neural function. Additionally, the exceptional regenerative ability of olfactory ensheathing glial cells makes them valuable research tools for drug screening targeting neural regeneration and examining potential neural tissue damaging substances.
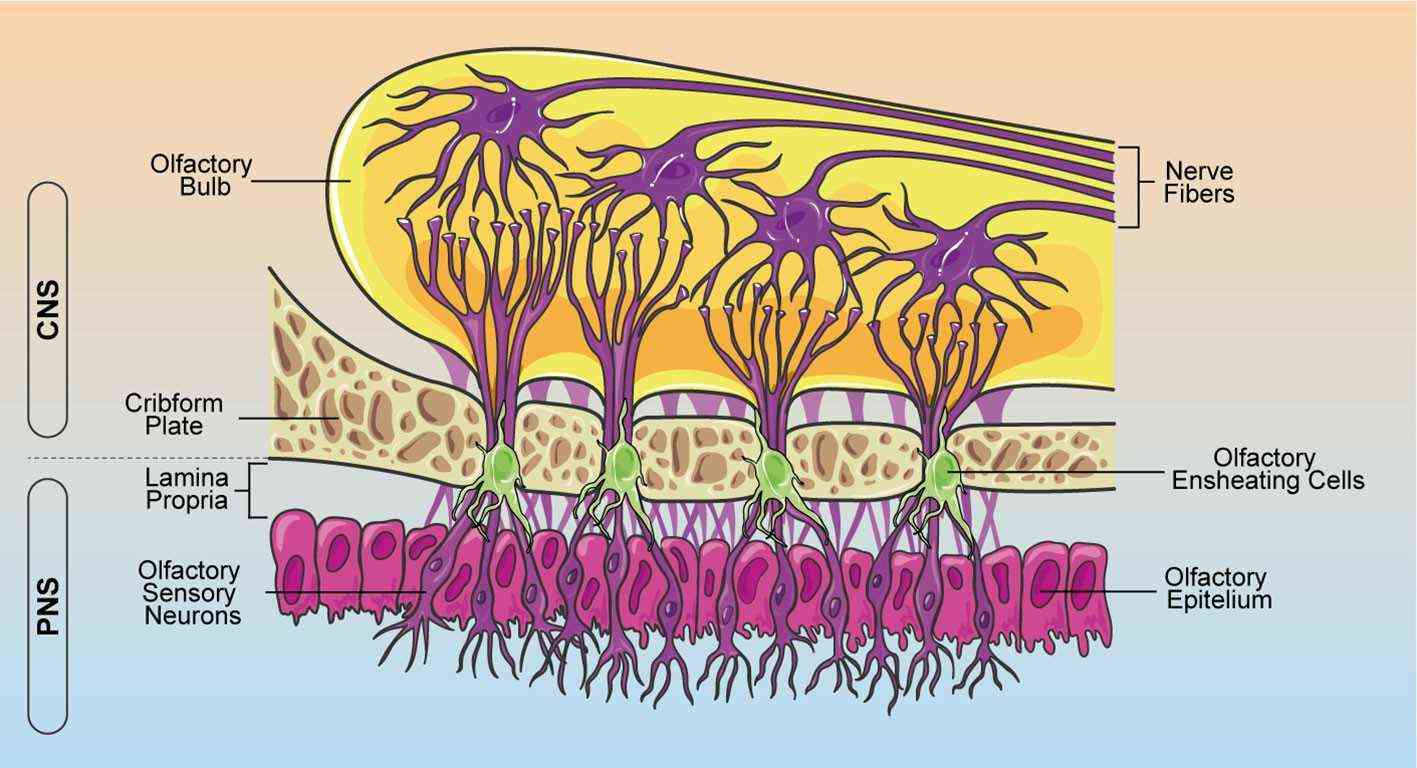 Fig. 1. Schematic representation of OEC localization within the olfactory system (Denaro S, D'Aprile S, et al., 2022).
Fig. 1. Schematic representation of OEC localization within the olfactory system (Denaro S, D'Aprile S, et al., 2022).
LPS Mediates Mitochondrial Oxidative Stress and Energy Disorder of Olfactory Ensheathing Glia
Olfactory ensheathing glia (OEG) are key to neuronal survival and regeneration, but their viability decreases with aging and chronic inflammation. The regulation of cell viability and organ homeostasis in response to stress depends significantly on mitochondrial function. He's team investigated how lipopolysaccharide (LPS)-induced inflammation affects immortalized OEG apoptosis, focusing on mitochondrial dysfunction and the JNK-Bnip3-Bax signaling pathway as potential mechanisms.
Mitochondrial damage triggers oxidative stress while simultaneously causing ATP depletion. Therefore, they investigated the link between mitochondrial damage and LPS-induced OEG apoptosis by analyzing mitochondrial redox balance and energy metabolism. Flow cytometry analysis with a mitochondrial ROS probe demonstrated that 5 μg/ml LPS elevated mitochondrial ROS levels beyond control measurements. The results from figures 1 a and b demonstrate that there is an excessive accumulation of ROS during inflammation. The treatment with 5 μg/ml LPS resulted in significant reductions of antioxidant levels including SOD, GSH and GPX (Fig. 1c–e). For mitochondrial energy metabolism, we measured ATP production, primarily generated by mitochondria. In comparison to the control, 5 μg/ml LPS significantly lowered ATP levels (Fig. 1f), showing ATP depletion under inflammation stress. ATP synthesis depends on the mitochondrial respiratory complex, which was analyzed via western blotting. LPS treatment downregulated its expression compared to the control (Fig. 1g–j), confirmed by qPCR (Fig. 1k–m). LPS also caused cyt-c release from mitochondria to the cytoplasm (Fig. 2A–C) and increased apoptosis, as shown by flow cytometry (Fig. 2D–E). Overall, LPS-induced inflammation leads to mitochondrial oxidative stress and respiratory complex downregulation.
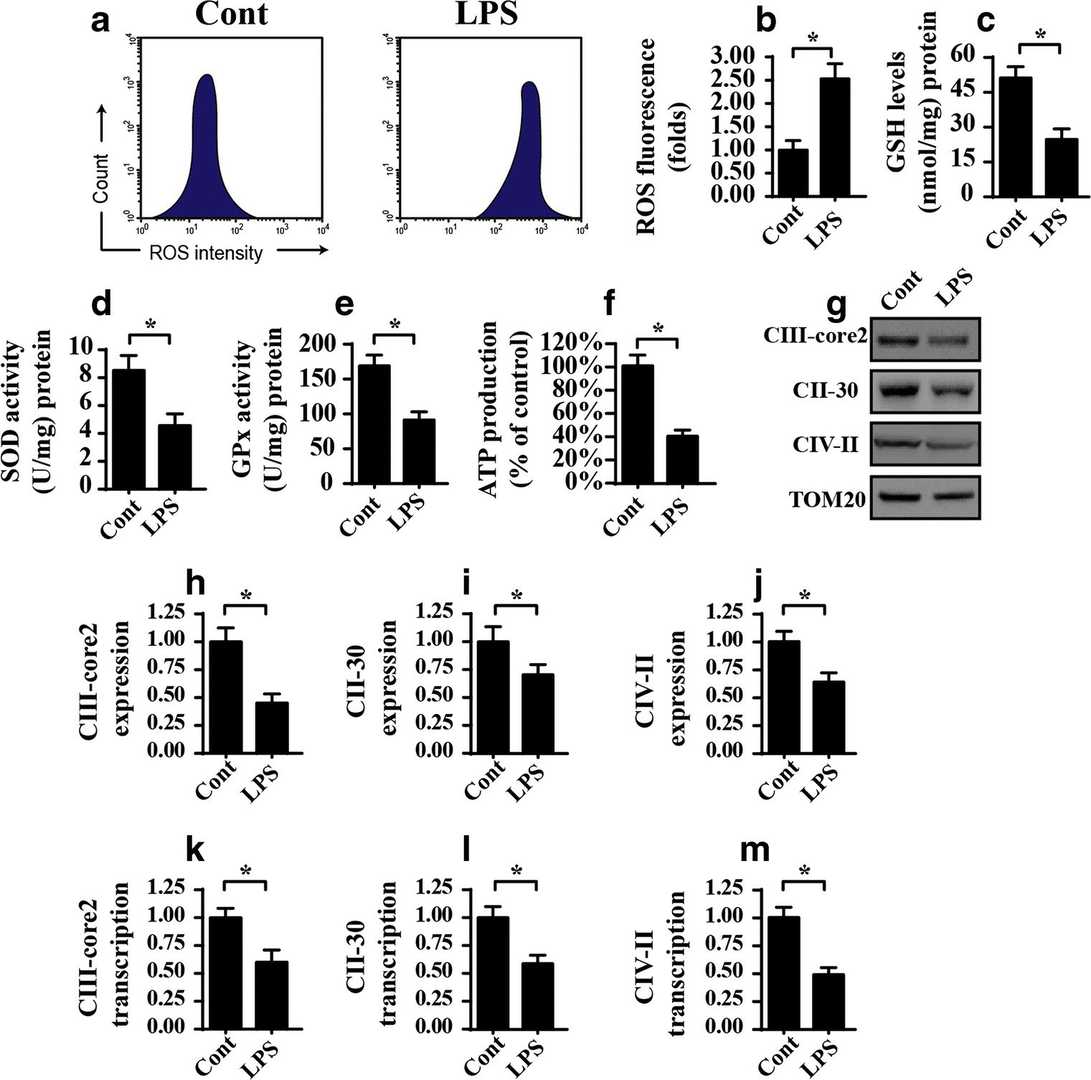 Fig. 1. LPS induces mitochondrial dysfunction as manifested by mitochondrial oxidative stress and mitochondrial respiratory complex downregulation in OEG (He M, Xiang Z, et al., 2019).
Fig. 1. LPS induces mitochondrial dysfunction as manifested by mitochondrial oxidative stress and mitochondrial respiratory complex downregulation in OEG (He M, Xiang Z, et al., 2019).
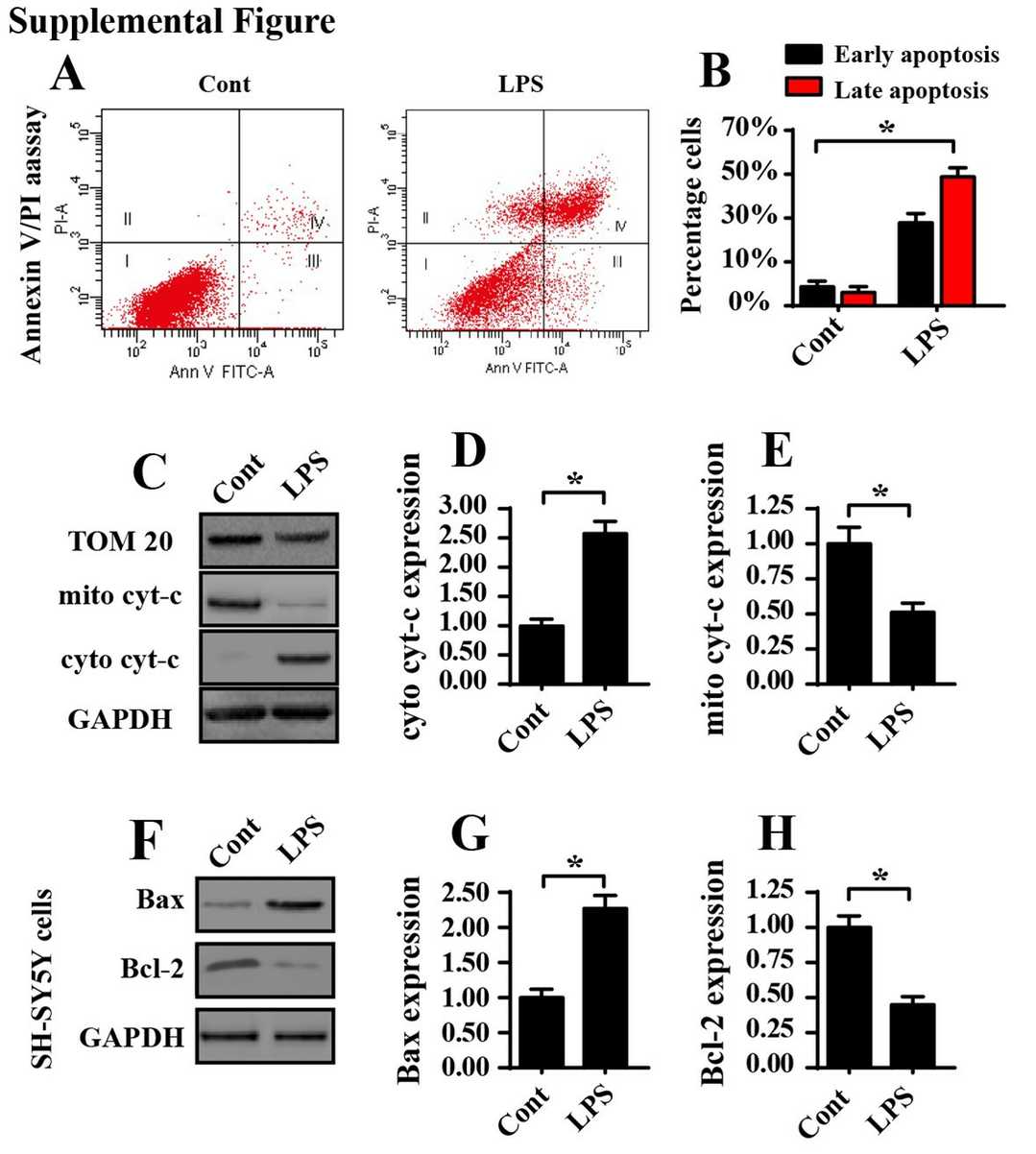 Fig. 2. The proapoptotic effect of LPS on OEG apoptosis (He M, Xiang Z, et al., 2019).
Fig. 2. The proapoptotic effect of LPS on OEG apoptosis (He M, Xiang Z, et al., 2019).
ZIKV Strains Replicate in Brain Endothelial and Neuroglial Cells
Zika virus (ZIKV) is associated with diseases like microcephaly and Guillain–Barré syndrome, with unclear neuroinvasion mechanisms. Mutso et al.examined the susceptibility of human and mouse neuroglial cells, including OECs and hCMEC/D3s, to various ZIKV strains.
They infected brain endothelial cells (hCMEC/D3) and immortalized neuroglial olfactory ensheathing cells (hOEC and mOEC) with three ZIKV strains (MR766, PRVABC59, and BeH819015) using an m.o.i. of 0.1. Vero cells served as a positive control. Vero cells exhibited maximum ZIKV replication (~107 p.f.u. ml?1 at 3 days p.i., Fig. 3a), brain endothelial and neuroglial cell lines also showed replication: highest in hCMEC/D3 (~106 p.f.u. ml?1 at 6 days p.i., Fig. 3b), followed by mOEC (~105 p.f.u. ml?1 at 2 days p.i., Fig. 3c). Within hOEC cells, the MR776 strain uniquely exhibited meaningful replication reaching approximately 104 p.f.u. ml?1 at 2 days p.i. (Fig. 3d). The MR766 strain exhibited faster replication rates compared to all other strains but mOEC. According to Figure 4a and 4b MR766 showed more significant cytopathic effects in Vero cells compared to other strains. Due to their intrinsic defense mechanisms neuroglial and endothelial cells showed resilience against both infection and toxic cell damage.
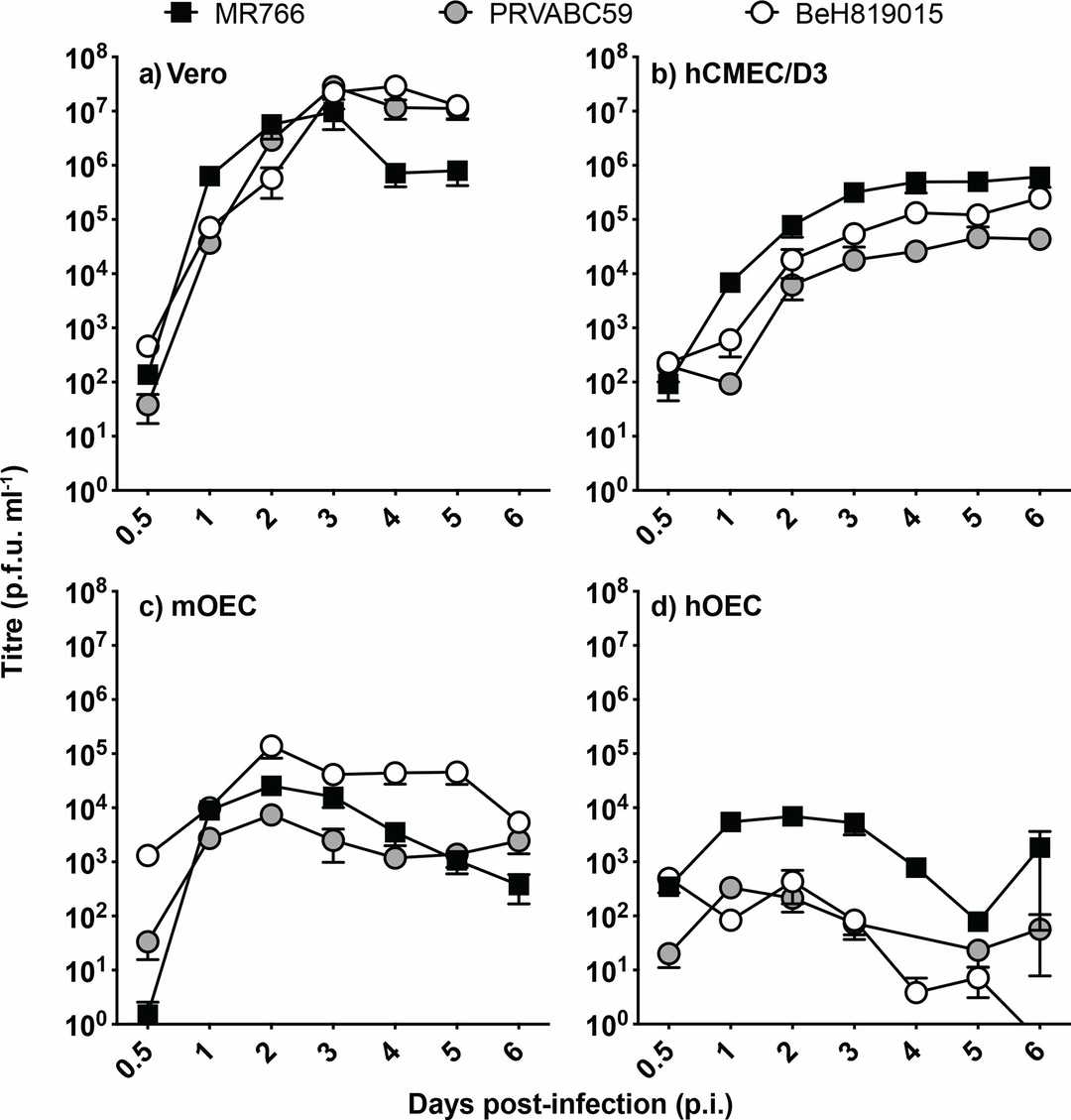 Fig. 3. ZIKV replication in non- neural and neuroglial cells (Mutso M, St John J A, et al., 2020).
Fig. 3. ZIKV replication in non- neural and neuroglial cells (Mutso M, St John J A, et al., 2020).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
