
Immortalized Human Microglia-SV40 (HMC-3)
Cat.No.: CSC-I2226Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Immortalized Human Microglia-SV40 (HMC-3) was developed from primary microglia from spinal cord and cortical tissues of 8- to 12-week-old embryos using SV40 virus-mediated immortalization. HMC3 cells grow in an adherent fashion with a morphology that is either macrophage-like or epithelial-like. In resting, these cells are more rounded with shorter protrusions and vacuolated cytoplasm. Additionally, some cells may appear spherical or bipolar in shape. When activated, these cells take on a different morphology and demonstrate increased phagocytic activity and migratory potential. HMC3 cells express numerous microglial-specific markers, such as CD68, CD11b, CD14, and Iba1, and they are negative for astrocyte marker GFAP as well as neuronal marker CD4. However, in the activated state, the cells can also express activation-related markers such as MHCII, CD68, and CD11b. The HMC3 cells also have standard microglial functions, which include phagocytosis, inflammatory response, and neurotransmitter regulation. These cells have been found to display phagocytic activity, cytokine secretion (such as IL-6), and neuroinflammatory response in vitro. As they are microglia-like cells from the central nervous system, the cell line is especially useful for studying neurodegenerative diseases (like Alzheimer's disease) and neuroinflammation.
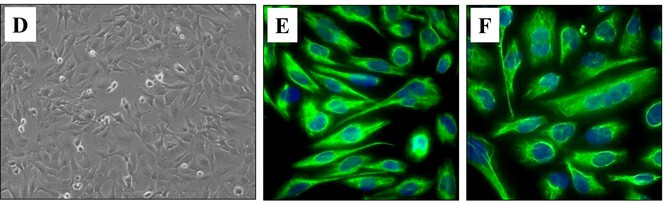 Fig. 1. (D) Phase contrast microscopy image of proliferating HMC3 cells (20X magnification). Immunofluorescence image of resting (E) and activated HMC3 cells (F) stained for vimentin cytoskeletal filaments with XNAMEX primary antibody and secondary mouse IgG antibody conjugated to CruzFluor488 (green fluorescence); nuclei stained with DAPI (blue fluorescence) (Ahuja S, Lazar L M, et al., 2021).
Fig. 1. (D) Phase contrast microscopy image of proliferating HMC3 cells (20X magnification). Immunofluorescence image of resting (E) and activated HMC3 cells (F) stained for vimentin cytoskeletal filaments with XNAMEX primary antibody and secondary mouse IgG antibody conjugated to CruzFluor488 (green fluorescence); nuclei stained with DAPI (blue fluorescence) (Ahuja S, Lazar L M, et al., 2021).
Mayaro and Una Viruses Efficiently Replicate in Human Microglia HMC3 Cells in a Time- and MOI-Dependent Manner
Mayaro (MAYV) and Una (UNAV) viruses are two alphaviruses that have recently emerged in the Americas. MAYV infection causes Mayaro fever, whereas the symptoms induced by UNAV remain unknown. In previous studies, MAYV was found to infect various types of human cells. However, the effect of MAYV on brain cells is not fully understood. Campos et al. investigated the susceptibility of two human brain cells, microglia HMC3 and brain microvascular endothelial HBEC-5i, to MAYV and UNAV infection using an inverted microscope, MTT assay, plaque-forming assays, and RT-qPCR.
To assess whether MAYV and UNAV can replicate in HMC3 or HBEC-5i cells, they performed a kinetic infection experiment. Immortalized human microglia HMC3 or HBEC-5i cells were infected with MAYV or UNAV at MOI 1 or 10, and viral progeny yields in the cell supernatants were quantified using plaque-forming assays at different time points. They found that MAYV and UNAV replicated in HMC3 cells in a time- and MOI-dependent manner (Fig. 1C and D). In contrast, only MAYV at a low level, but not UNAV, infected HBEC-5i cells (Fig. 1E). They confirmed these results by evaluating the expression of E1 and nsP1 viral proteins in infected cells using immunofluorescence assays and Western blot. Strong expression of both proteins was detected in MAYV- and UNAV-infected HMC3 cells at 48 hpi (Fig. 2A), and high protein levels were confirmed using Western blot (Fig. 2B and C). In contrast, low protein expression was detected in MAYV-infected HBEC-5i cells and no expression in UNAV-infected HBEC-5i cells (Fig. 2A, D, E). Taken together, these data show that MAYV and UNAV efficiently infect human microglia HMC3 cells.
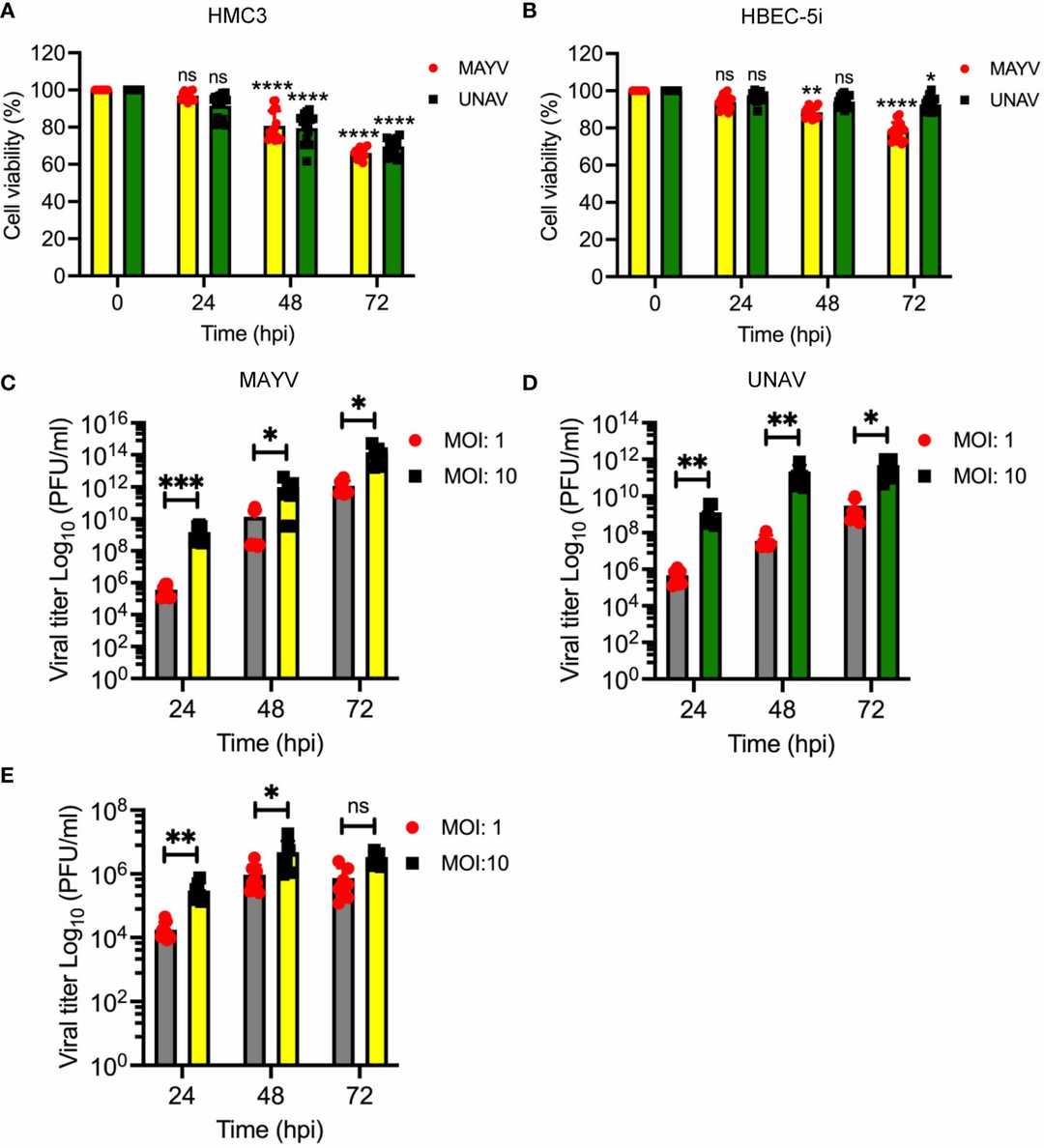 Fig. 1. Mayaro and Una viruses efficiently infect human microglia HMC3 cells (Campos D, Sugasti-Salazar M, et al., 2024).
Fig. 1. Mayaro and Una viruses efficiently infect human microglia HMC3 cells (Campos D, Sugasti-Salazar M, et al., 2024).
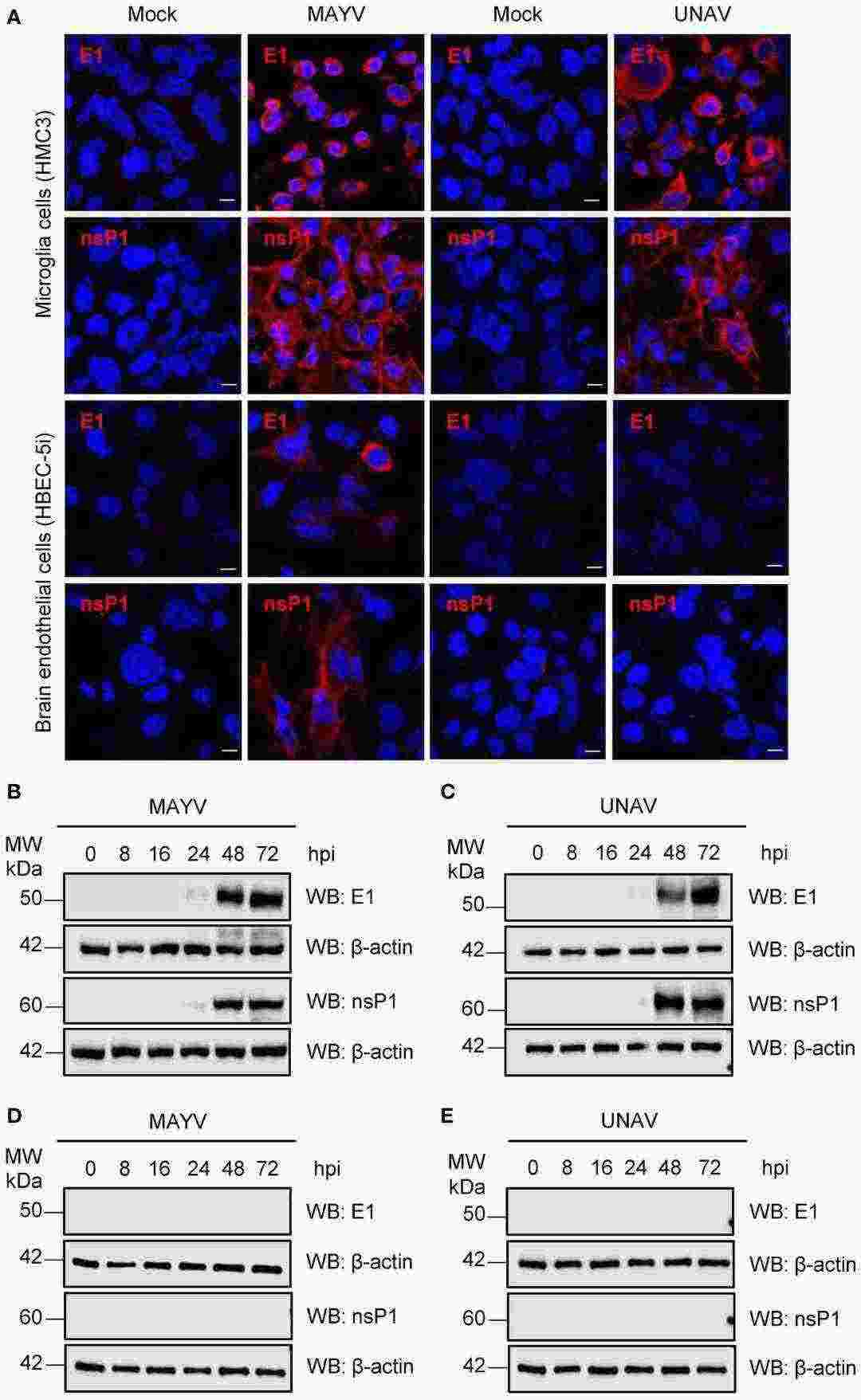 Fig. 2. Detection of viral E1 and nsP1 proteins in MAYV- or UNAV-infected HMC3 and HBEC-5i cells (Campos D, Sugasti-Salazar M, et al., 2024).
Fig. 2. Detection of viral E1 and nsP1 proteins in MAYV- or UNAV-infected HMC3 and HBEC-5i cells (Campos D, Sugasti-Salazar M, et al., 2024).
LAV-BPIFB4-infected STHdh Q111/111 Displays an Anti-Inflammatory and Pro-Resolving M2-Polarizing Effect on HM-SV40
Huntington's disease (HD) is a neurodegenerative disorder characterized by neural death due to expanded CAG repeats in the huntingtin protein (Htt). Neuroinflammation plays a key role in HD progression. BPIFB4 is a longevity-associated variant (LAV) that is overrepresented in centenarians and has been demonstrated to have immunomodulatory properties. Pardo et al. investigated the potential of the LAV-BPIFB4 protein to counteract the progression of HD by improving striatal–microglia communication and thus explore a potential therapeutic approach against the disease.
Their previous work showed that LAV-BPIFB4-treated mice had elevated SDF-1 levels in peripheral blood and myocardium, which was crucial for LAV-BPIFB4-induced benefits. Since BPIFB4 had a macrophage M2-polarizing effect via a CXCR4-dependent mechanism, we next examined SDF-1 secretion after BPIFB4 infection. ELISA assays confirmed that STHdh Q111/111 cells infected with LAV-BPIFB4, but not WT-BPIFB4, secreted higher SDF-1 levels compared with the untreated cells (Fig. 3a). Next, conditioned medium (CM) from these cells was used to treat immortalized human microglia-SV40 microglial cells in vitro. Flow cytometry analysis revealed that the normal STHdh Q7/7 cells induced the upregulation of CD163 on HM-SV40 cells, while the mutant STHdh Q111/111 cells failed to exert this effect. Interestingly, after 48-h treatment with CM from LAV-BPIFB4-infected STHdh Q111/111 cells, CD163 expression and IL-10 release significantly increased (Fig. 3b - d). This effect was inhibited by the CXCR4 antagonist AMD3100 (20 μM), which confirmed the CXCR4-dependent mechanism (Fig. 3b - d).
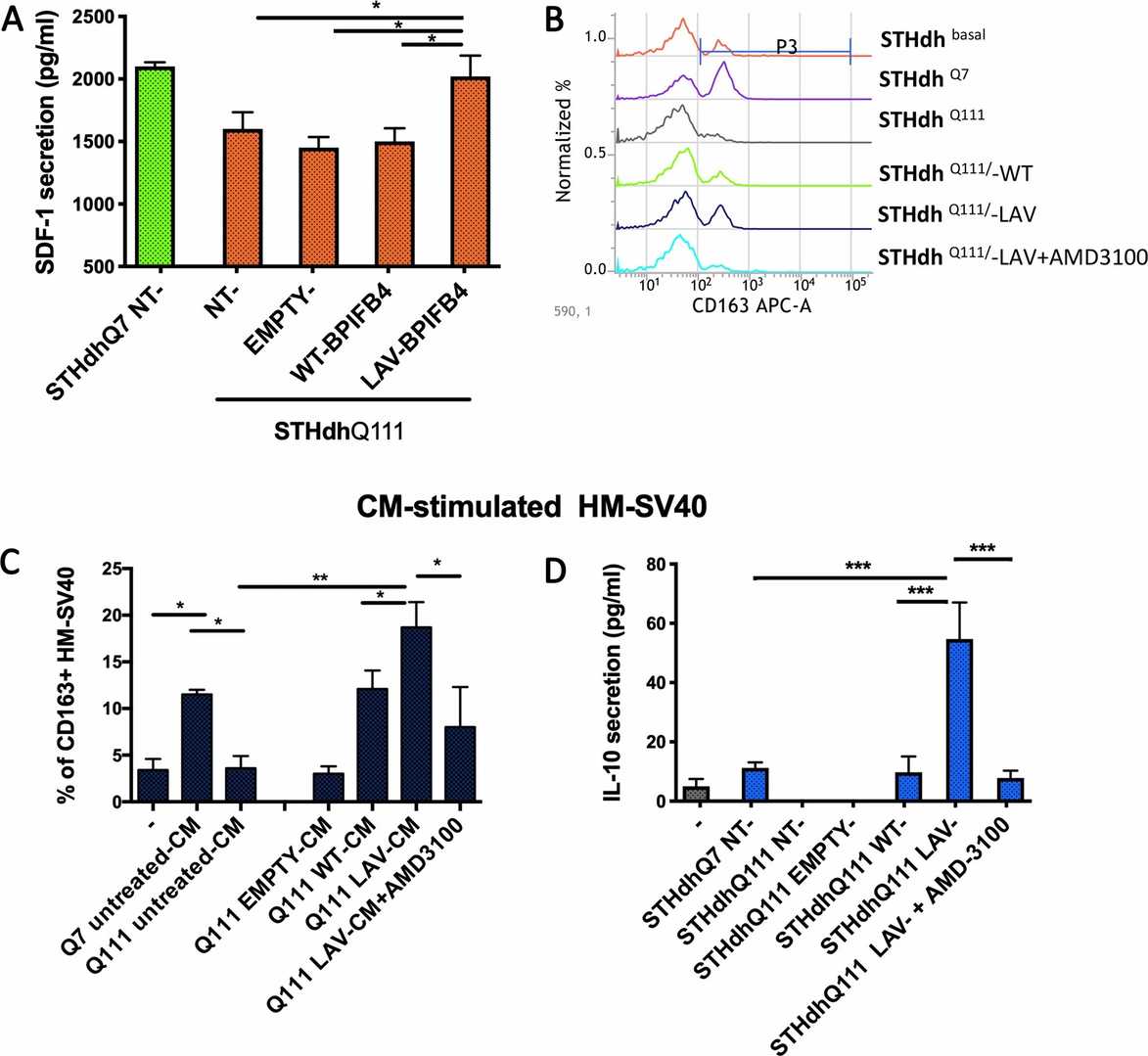 Fig. 3. Analysis of anti-inflammatory and M2-polarizing action of CM from LAV-BPIFB4-infected striatal cells (Pardo A Di, Ciaglia E, et al., 2020).
Fig. 3. Analysis of anti-inflammatory and M2-polarizing action of CM from LAV-BPIFB4-infected striatal cells (Pardo A Di, Ciaglia E, et al., 2020).
Resting HMC3 cells were strongly positive for the microglia/macrophage marker IBA1, positive for the endotoxin receptor CD14, but negative for the astrocyte marker GFAP. Markers of activated microglia, namely MHCII, CD68 and CD11b were negative in resting HMC3 cells, but upregulated after activation by IFN-gamma (10 ng/ml, 24 h).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
