
Immortalized Human Bone Marrow Mesenchymal Cells-hTERT
Cat.No.: CSC-I9085L
Species: Homo sapiens
Source: Bone Marrow
Morphology: spindle-shaped
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
The "immortalized human bone marrow mesenchymal cells-hTERT" cell line is an immortalized bone marrow mesenchymal stem cell (MSC) line achieved through the transfection of the telomerase reverse transcriptase (hTERT) gene. The cell line originates from human bone marrow which functions as a critical hematopoietic organ found in bones including ribs, spine, and sternum. The main functional features of original mesenchymal stem cells persist in immortalized bone marrow mesenchymal stem cells. For instance, when exposed to osteogenic induction conditions these cells can produce calcified nodules and express type I collagen while chondrogenic conditions lead to the expression of type II collagen and adipogenic conditions result in lipid droplet accumulation. Additionally, the hMSC-TERT cells show self-renewal capabilities and greatly enhanced telomerase activity which results in prolonged cell lifespan. These cells also express specific markers including CD44 and CD73 (alkaline phosphatase) while showing positive toluidine blue staining which indicates chondrocytic properties.
The multipotent differentiation potential and stable biological characteristics of hTERT-MSCs make them good choices for tissue engineering applications including bone and cartilage fabrication for fracture repair and treatment of skeletal injuries. These cells support the treatment of blood disorders like leukemia by promoting the development and specialization of hematopoietic stem cells. Moreover, hMSC-TERT cells function as an experimental model to explore stem cell biology and molecular regulatory networks associated with diseases in addition to cellular aging mechanisms.
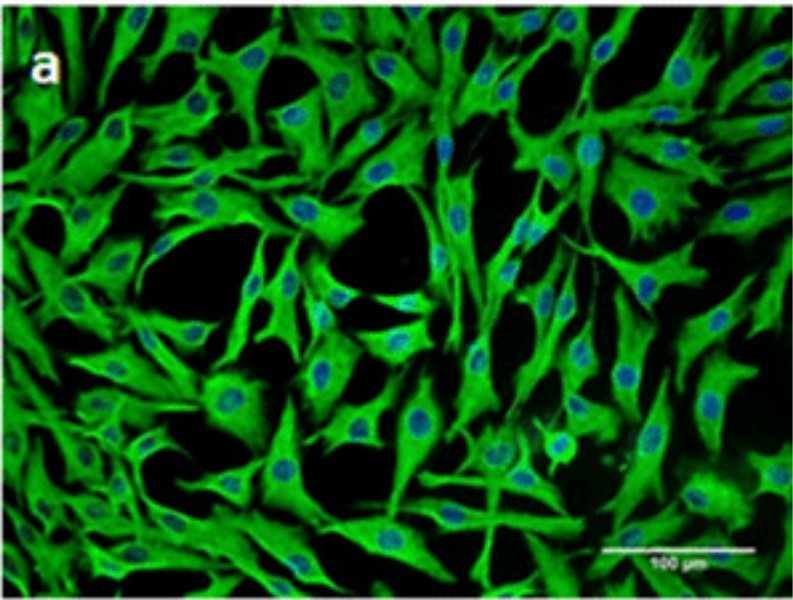 Fig. 1. The human bone marrow stromal (mesenchymal) stem cell (hMSC) immortalized with human telomerase reverse transcriptase gene (hMSC-TERT) (AI-Nbaheen M, Vishnubalaji R, et al., 2012).
Fig. 1. The human bone marrow stromal (mesenchymal) stem cell (hMSC) immortalized with human telomerase reverse transcriptase gene (hMSC-TERT) (AI-Nbaheen M, Vishnubalaji R, et al., 2012).
The p53 Isoform Delta133p53 Drives High Level of HGF Secretion in Ewing Sarcoma
Ewing sarcoma (EWS) represents a challenging cancer with limited success in current treatments due to its aggressive nature and poor prognosis in advanced stages. Aberrant expression of HGF, driven by delta133p53, facilitates tumor growth and metastasis. To address this, Charan et al.explored the therapeutic potential of combining an HGF receptor neutralizing antibody (AMG102) with GD2-directed CAR-T therapy.
They examined the underlying molecular mechanism of high levels of HGF expression in EWS. Ewing sarcoma (EWS) is characterized by the expression of the EWS/FLI fusion protein, a key driver mutation disrupting gene regulation related to cancer. They hypothesized that EWS/FLI leads to high HGF expression. To test this, they used siRNA to knock down EWS/FLI1 in cell lines and analyzed HGF expression. Surprisingly, as shown in Figure 1a, HGF levels remained unchanged, suggesting its regulation is independent of EWS/FLI. In Ewing sarcomas, only 10% show genetic lesions in TP53, with most expressing functional wild-type p53. Surprisingly, the oncogenic p53 isoform Δ133p53 is abnormally expressed in EWS. They investigated whether Δ133p53 influences HGF expression using Ewing cell lines ES2 and ES4 with inducible vectors to inhibit Δ133p53. Figures 1b and 1c show that reducing Δ133p53 significantly decreased HGF levels. To confirm their findings further, they genetically engineered immortalized human bone marrow mesenchymal cells (hMSC-hTERT), thought to be the cellular origin of EWS, by overexpressing Δ133p53 or EWS/FLI. Overexpressing Δ133p53 in hMSC-hTERT increased HGF expression, unlike EWS/FLI (Fig.1f-g). Taken together, their data demonstrate that high level of HGF expression and secretion are driven by Δ133p53 and independent of EWS/FLI1 expression.
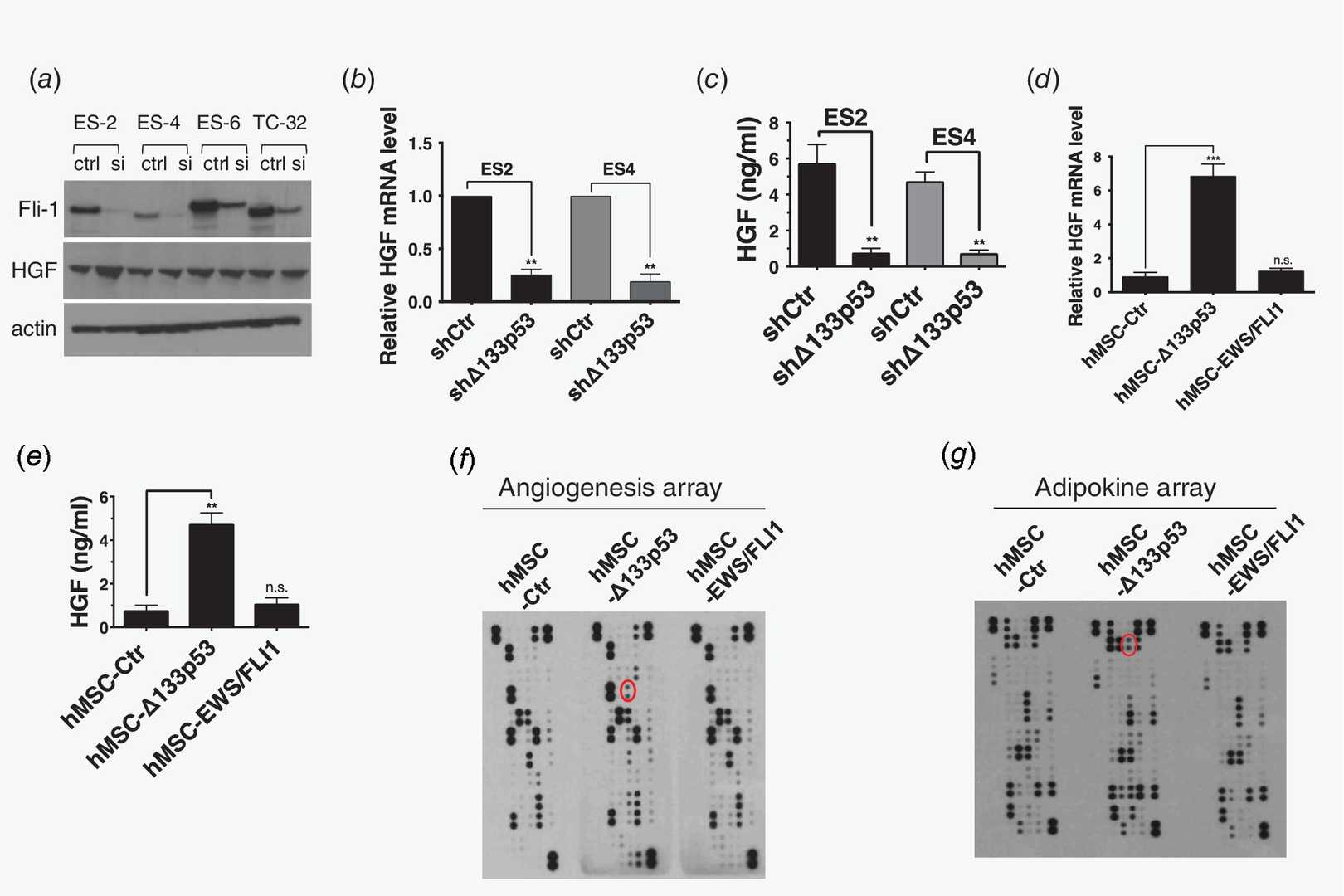 Fig. 1. Δ133p53 expression drives HGF expression and secretion (Charan M, Dravid P, et al., 2019).
Fig. 1. Δ133p53 expression drives HGF expression and secretion (Charan M, Dravid P, et al., 2019).
MMC Stabilized MVNs in Microfluidic Devices
Microvessels are key in molecular exchange and disease processes. Current two-dimensional and in vivo models lack detailed spatiotemporal insights. While engineered MVNs in microfluidics show promise, their instability limits long-term studies. Wan et al. introduces macromolecular crowding (MMC) in microfluidic devices as a method to stabilize MVNs by mimicking naturally crowded in vivo conditions, thereby obviating the need for auxiliary cells or protease inhibitors.
Utilizing a design with three channels separated by permeable partitions (Fig. 2A), HUVECs and immortalized human bone marrow mesenchymal cells – hTERT (hTERT-MSCs), acting as pericytes, were seeded into the central channel in a fibrinogen solution (Fig. 2B). Thrombin facilitated rapid fibrin hydrogel formation, serving as a 3D matrix for the cells. Protease inhibitors were excluded to avoid affecting biological processes. Cells formed tubule-like structures and interconnected MVNs within two days (Fig. 2C). One day post-seeding, macromolecular crowding (MMC) was optionally added to the culture medium. The MMC cocktail, using two sizes of Ficoll macromolecules, has shown to promote ECM deposition in both 2D and 3D cultures.
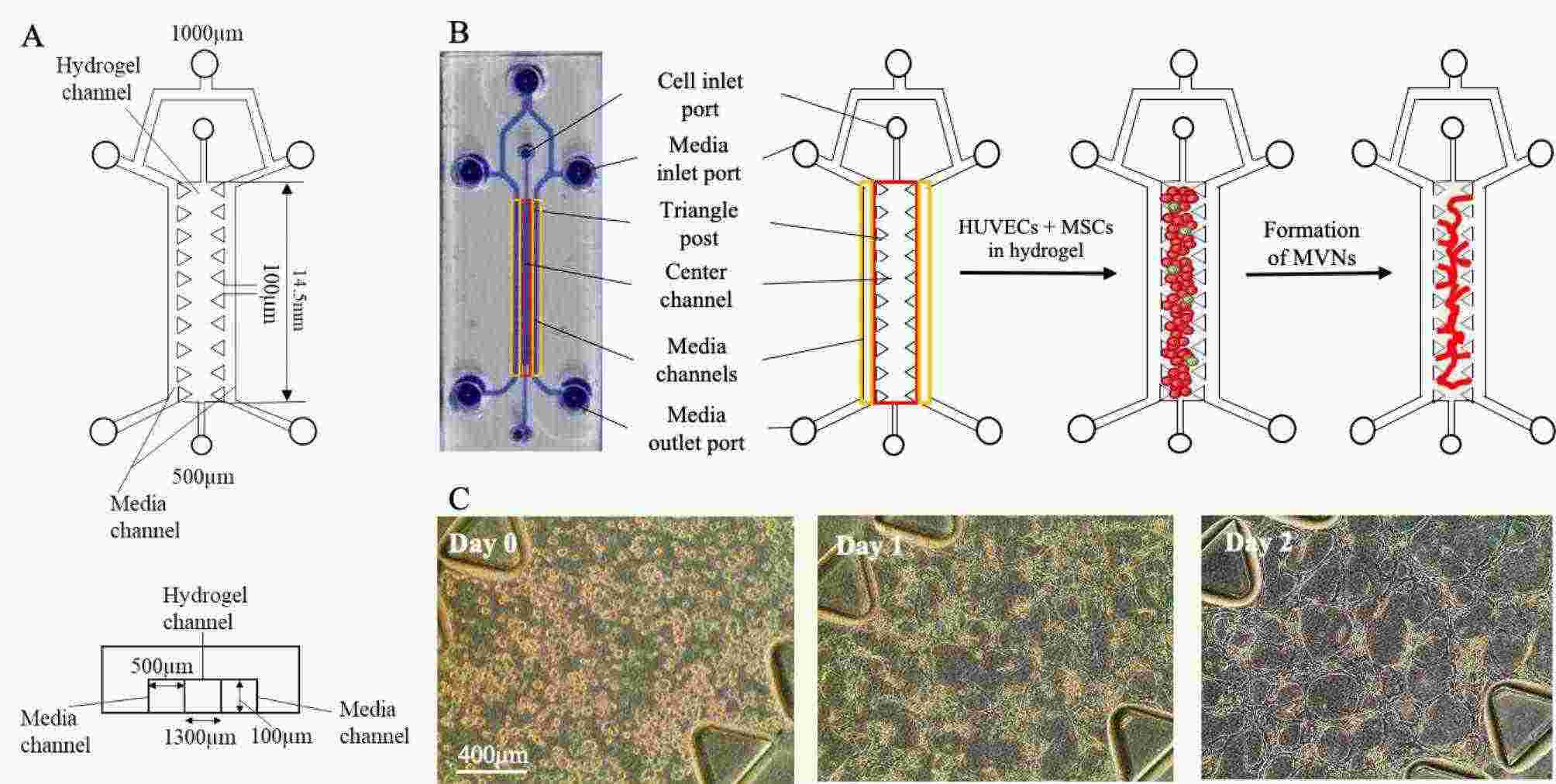 Fig. 2. HUVECs and MSCs form interconnected MVNs within the microfluidic device (Wan H Y, Chen J C H, et al., 2023).
Fig. 2. HUVECs and MSCs form interconnected MVNs within the microfluidic device (Wan H Y, Chen J C H, et al., 2023).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

