
Immortalized Human Astrocytes, fetal-SV40
Cat.No.: CSC-I9065L
Species: Homo sapiens
Source: Fetal Brain
Morphology: Multipolar
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Astrocytes are the major cell type and among the most functionally diverse group of cells in the mammalian brain. They provide a variety of supportive functions to their partner neurons in the central nervous system (CNS), such as neuronal guidance during development, and nutritional and metabolic support throughout life. Much of what we have learned about astrocytes is from in vitro studies, and astrocyte culture is a valuable tool for exploring the diverse properties of this cell type. They are also highly useful for establishing disease models for high-throughput and high-content screening.
Immortalized Human Astrocytes, fetal-SV40, are an important in vitro model system developed to overcome the limitations of primary human astrocytes. Current research applications heavily leverage their advantages:
1) Blood-Brain Barrier (BBB) Modeling: They are a key component of co-culture BBB models (with endothelial cells and pericytes) used to study barrier integrity, transporter function, and mechanisms of drug delivery or neurotoxicity in the CNS.
2) Neuroinflammation Studies: Their robust response to cytokines makes them ideal for investigating astrocyte reactivity, signaling pathways, and the release of inflammatory mediators in contexts like neurodegeneration, infection, or trauma.
3) Neurodegenerative Disease Modeling: Used to study astrocytic contributions to diseases like Alzheimer's, Parkinson's, and amyotrophic lateral sclerosis (ALS), with a particular focus on neuroinflammation, glutamate handling, and neuroprotective/neurotoxic roles.
4) Neurotropic Infection Research: Employed to model interactions with viruses and other pathogens, studying viral entry, replication as well as astrocyte-mediated immune responses within the CNS.
5) Neuropharmacology & Toxicological Screening: Their scalability enables high-throughput screening of potential neuroprotective drugs or assessment of compound toxicity on glial cells.
The immortalized human astrocytes provided by Creative Bioarray are developed by immortalizing primary human cortical astrocytes with SV40 Large T antigen. Creative Bioarray also offers optimized media and reagents for the growth of immortalized human astrocytes, which are quality tested together and guaranteed to give maximum performance for in vitro culture.
Curcumin Induces Apoptosis and Necrosis in Human Brain Glioma Cell Line (CCF) and SV40-Immortalized Normal Human Astrocytes (INHA)
Glioblastoma is an aggressive brain tumor with limited treatment options and significant resistance to conventional therapies. This study explored the effects of combining curcumin treatment with clusterin inhibition on cell death in glioma cells.
The results showed that the combination of clusterin silencing and curcumin treatment induces cell death. This combination therapy significantly elevated reactive oxygen species (ROS), triggering oxidative stress, which acted as a key upstream mediator of apoptosis. Elevated ROS levels were found to be associated with caspase activation, suggesting apoptosis as the primary mode of cell death. Furthermore, autophagy was induced as a complementary mechanism, with upregulation of LC3B contributing to the enhanced cytotoxic effects.
The synergy between clusterin knockdown-induced senescence and curcumin's pro-apoptotic and pro-autophagic effects highlights a potential novel therapeutic strategy for gliomas. These findings underscore the potential of this combination therapy in overcoming glioma resistance and improving treatment outcomes by dually inducing oxidative stress and cell death pathways.
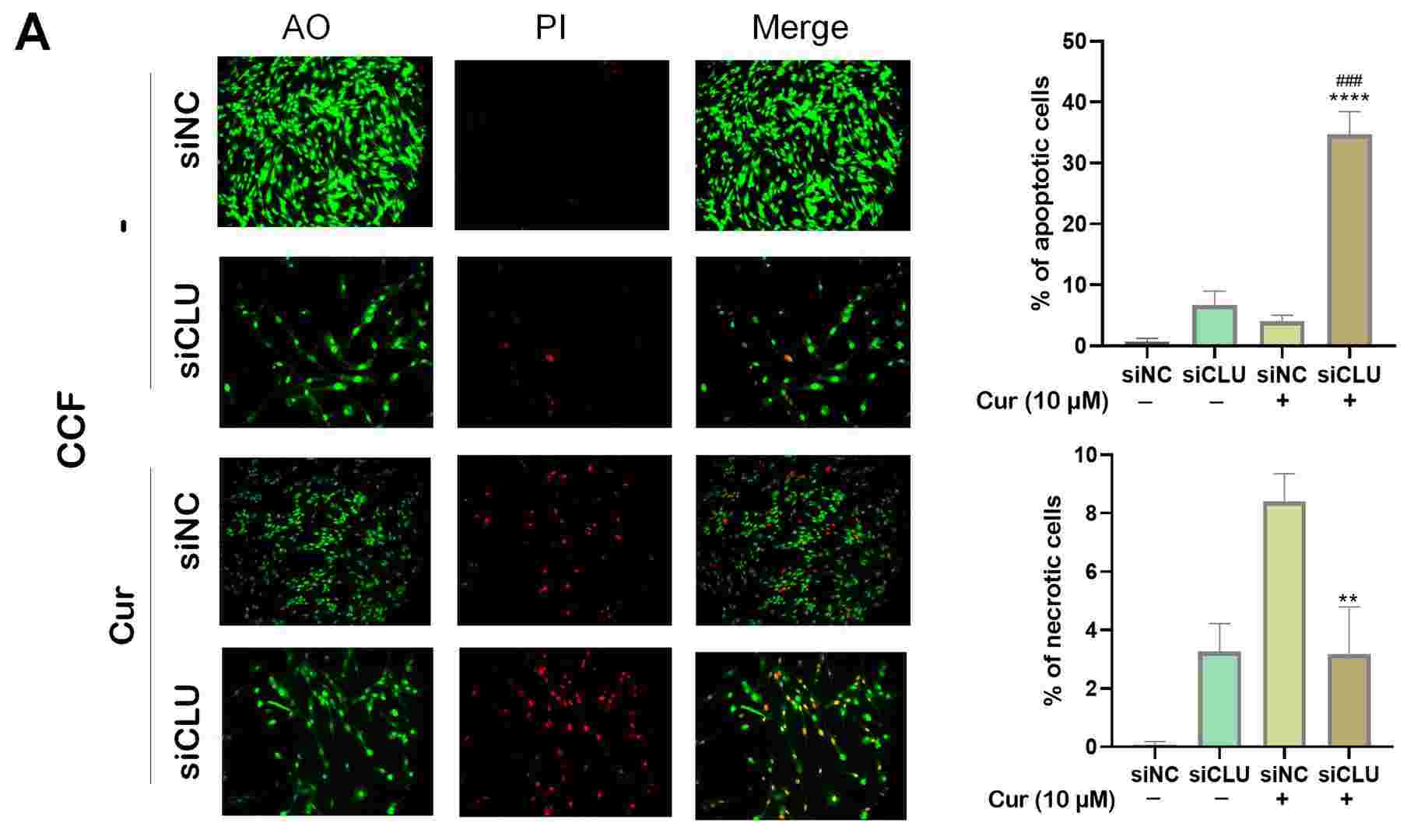 Fig. 1. Monitoring cell death processes after curcumin treatment (Sultana, Pinky, and Jiri Novotny. 2025).
Fig. 1. Monitoring cell death processes after curcumin treatment (Sultana, Pinky, and Jiri Novotny. 2025).
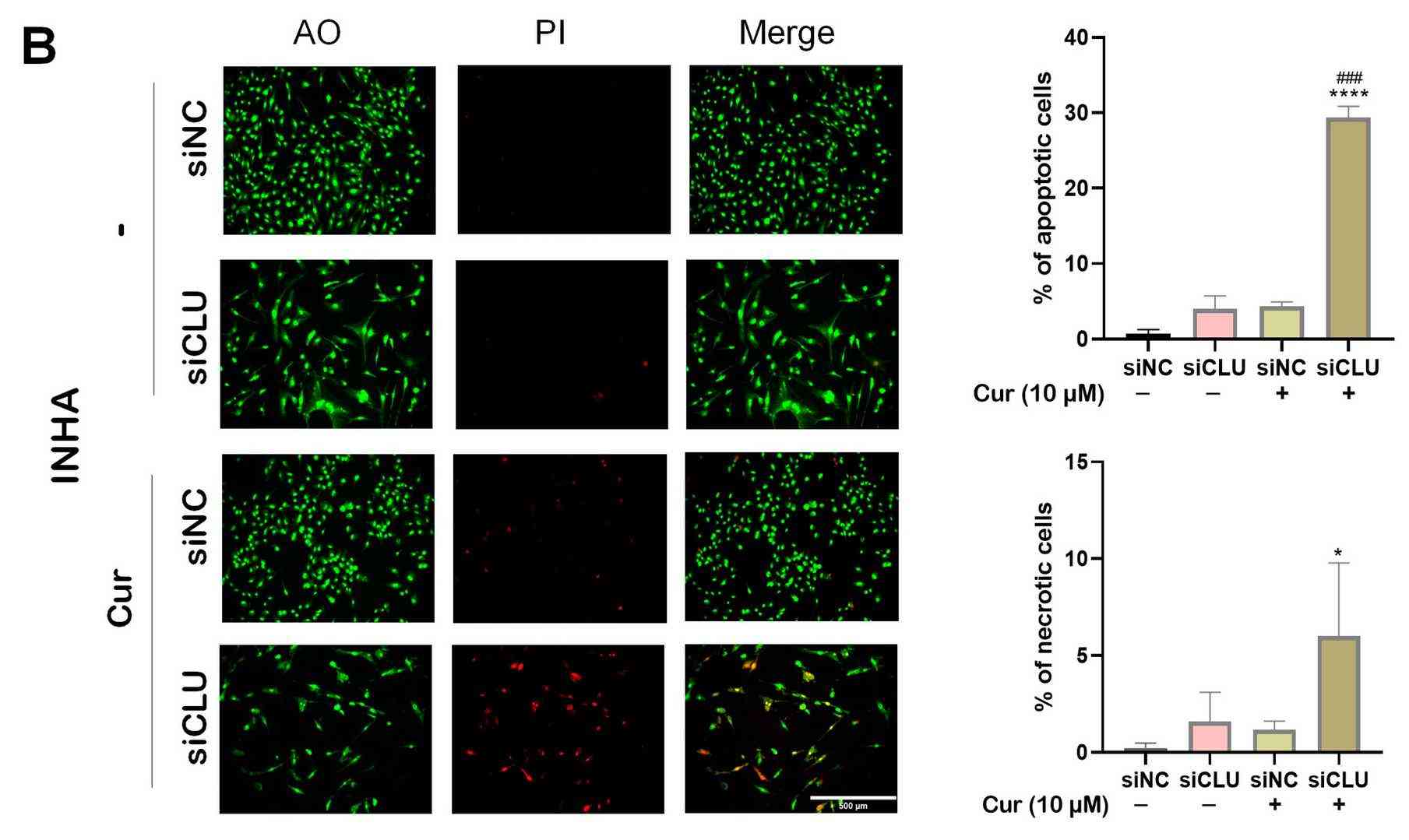 Fig. 2. Effect of curcumin on autophagy (Sultana, Pinky, and Jiri Novotny. 2025).
Fig. 2. Effect of curcumin on autophagy (Sultana, Pinky, and Jiri Novotny. 2025).
In Situ Monitoring of the Formation and Maturation of a Sensorized Microfluidic BBB Model
A significant challenge in the treatment of central nervous system (CNS) disorders lies in the blood–brain barrier (BBB). In vivo models are constrained by high costs, lengthy experimental timelines, ethical concerns, and interspecies variations. In vitro models, particularly microfluidic BBB-on-a-chip devices, have been developed to address these limitations. In this framework, Ceccarelli, Maria Cristina, et al. developed an innovative microfluidic system that integrates thin-film electrodes for non-invasive, real-time monitoring of BBB integrity using electrochemical impedance spectroscopy (EIS).
The model incorporates human endothelial cells in a vessel-like arrangement to mimic the vascular component and three-dimensional cell distribution of human astrocytes and microglia to simulate the parenchymal compartment. The device demonstrated successful BBB formation and maturation, confirmed through live/dead assays, immunofluorescence and permeability assays.
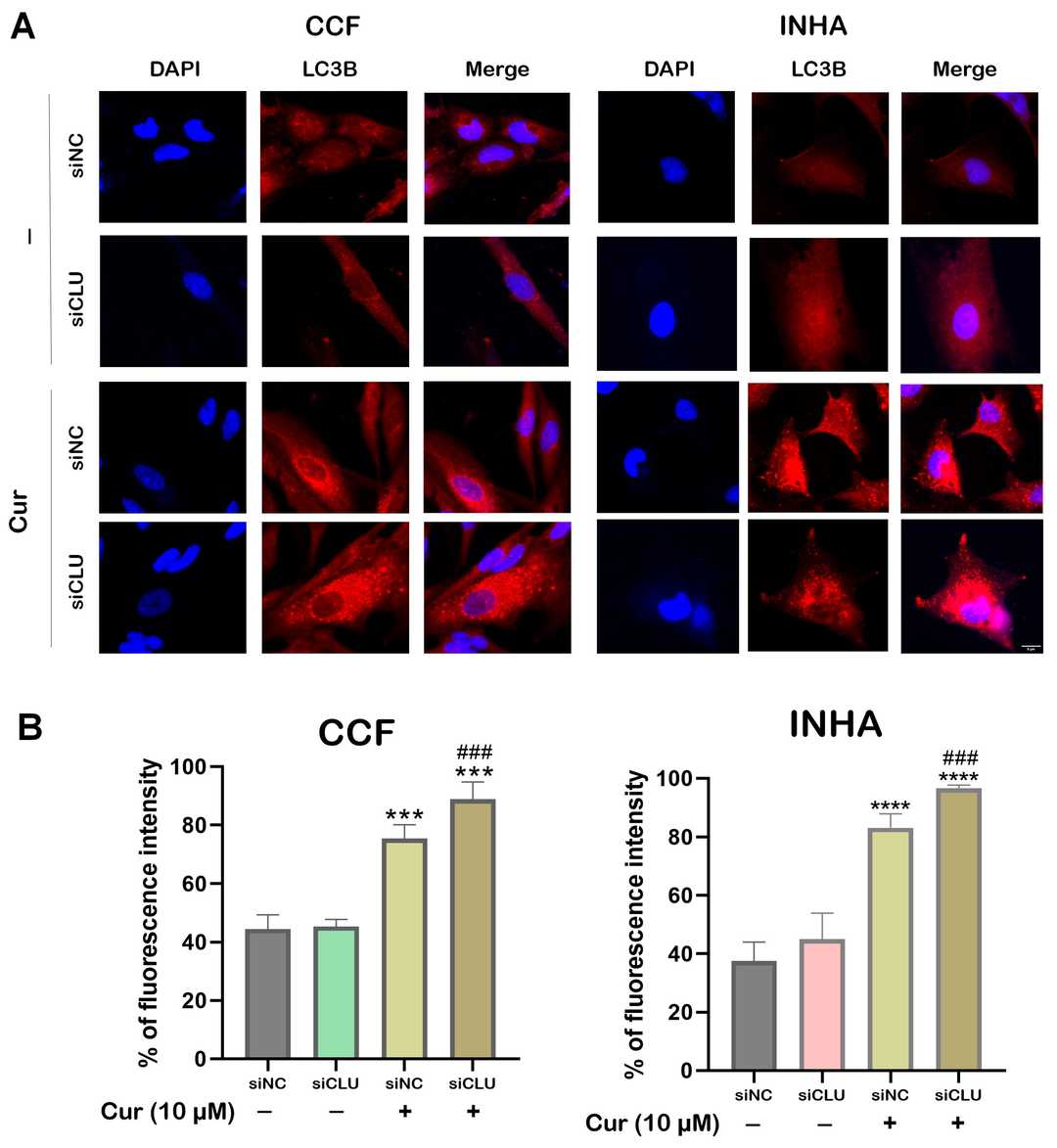 Fig. 3. Confocal microscopy images showing live/dead cell staining and cell distribution in the microfluidic device (Ceccarelli, Maria Cristina, et al. 2024).
Fig. 3. Confocal microscopy images showing live/dead cell staining and cell distribution in the microfluidic device (Ceccarelli, Maria Cristina, et al. 2024).
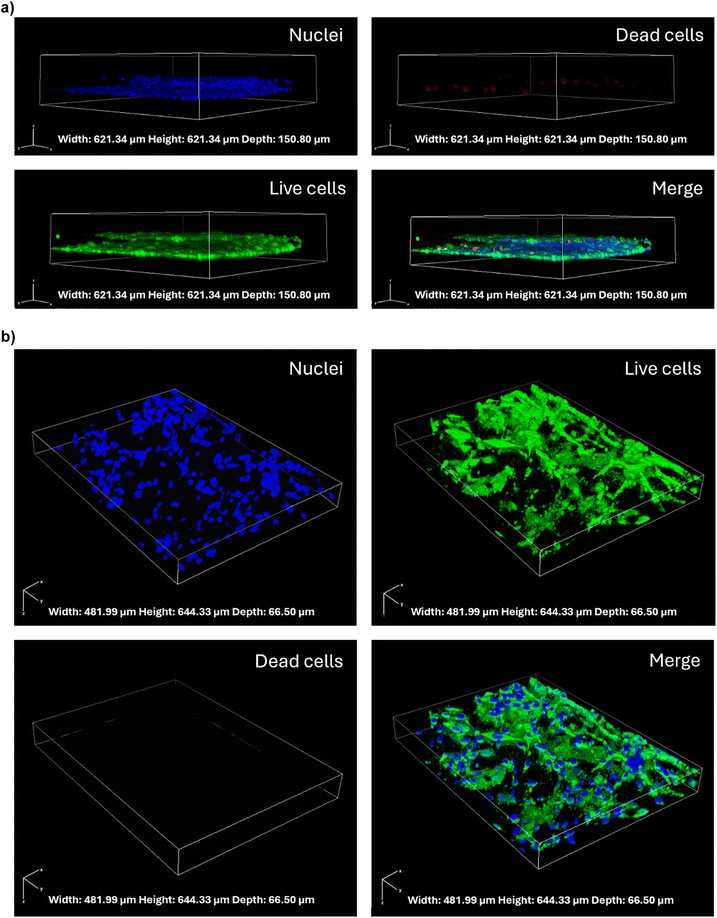 Fig. 4. Confocal microscopy images showing immunostaining and cell distribution in the microfluidic device (Ceccarelli, Maria Cristina, et al. 2024).
Fig. 4. Confocal microscopy images showing immunostaining and cell distribution in the microfluidic device (Ceccarelli, Maria Cristina, et al. 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

