Human Dermal Fibroblasts
Cat.No.: CSC-C4849L
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human dermal fibroblasts (HDF) are mesenchymal/stromal cells derived from the embryonic mesoderm. They reside in the dermal layer of the skin, where they produce extracellular matrix (ECM) proteins to strengthen the dermal compartment and interact with epidermal cells. In response to skin injury, HDF acquire myofibroblast phenotype characterized by increased proliferation, migration and ECM synthesis activity. Therefore, HDF plays a central role in skin wound healing.
In vitro cultured HDF adhere to the bottom of the culture dishes and proliferate. HDF do not need immortalization steps before the culture, maintaining the native genetic background of the patient. Furthermore, after several passages, HDF loose the epigenetic signature associated with hormonal, nutritional and treatment state of the patient. Thus, HDF is an interesting tool for linking genetics with pathophysiology and studying therapeutic responses in vitro. Moreover, this model served for the diagnosis and investigation of diseases. For instance, HDF were used to study impairments in the wound healing process, skin fibrosis propensity in response to radiotherapy, oxidative phosphorylation dysfunction, and to assess the presence or the risk of metabolic diseases in patients.
Long Noncoding RNAs H19 and PURPL Affects Cell Senescence in Human Dermal Fibroblasts
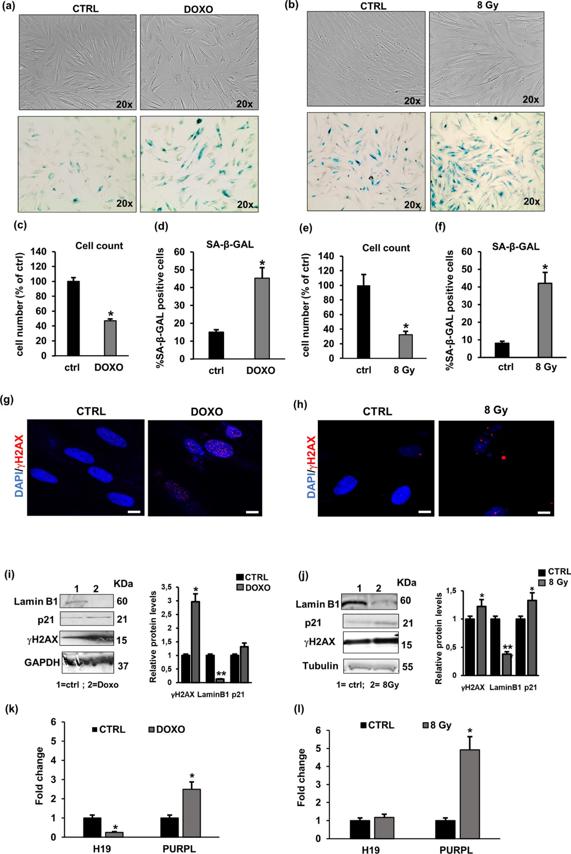
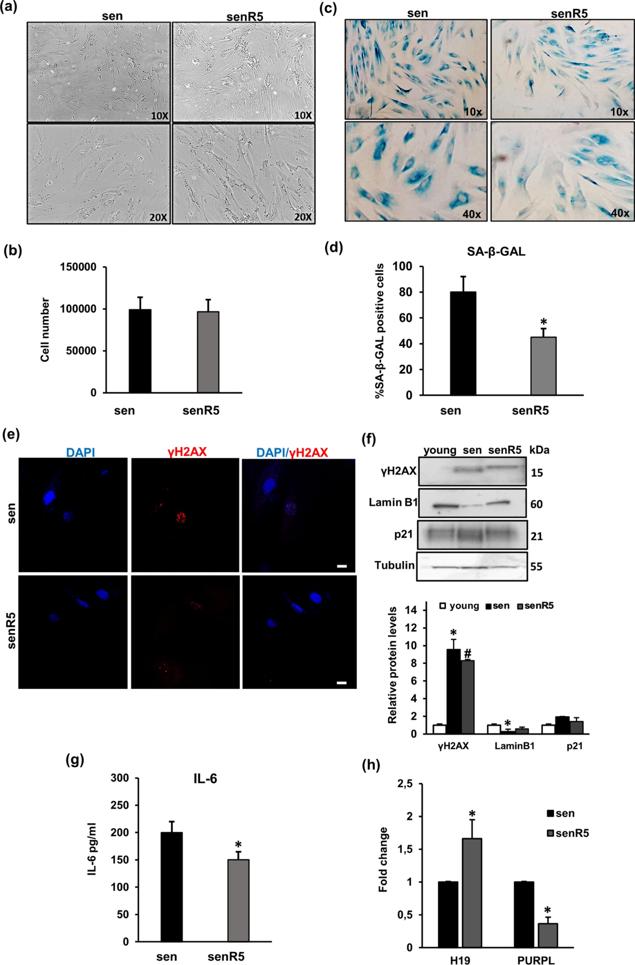
Cellular senescence is a permanent cell growth arrest that occurs in response to various intrinsic and extrinsic stimuli and is accompanied by cellular and molecular changes. Long non-coding RNAs (lncRNAs) are key regulators of cellular senescence by affecting the expression of many important genes involved in senescence-associated pathways and processes.
Here, Elena Frediani s team evaluated a panel of lncRNAs associated with senescence for their differential expression between young and senescent human dermal fibroblasts (NHDFs) and studied the effect of a known senomorphic compound, resveratrol, on the expression of lncRNAs in senescent NHDFs. As markers of senescence, cell growth, senescence-associated (SA)-β-Gal staining, and the expression of p21, Lamin B1 and γH2AX were assessed. They found that H19 and PURPL were the most altered lncRNAs in replicative, in doxorubicin (DOXO) and ionising radiation (IR)-induced senescence models, highlighting the role of H19 and PURPL in senescent phenotype and suggested that these lncRNAs may be important for senescence-related diseases.
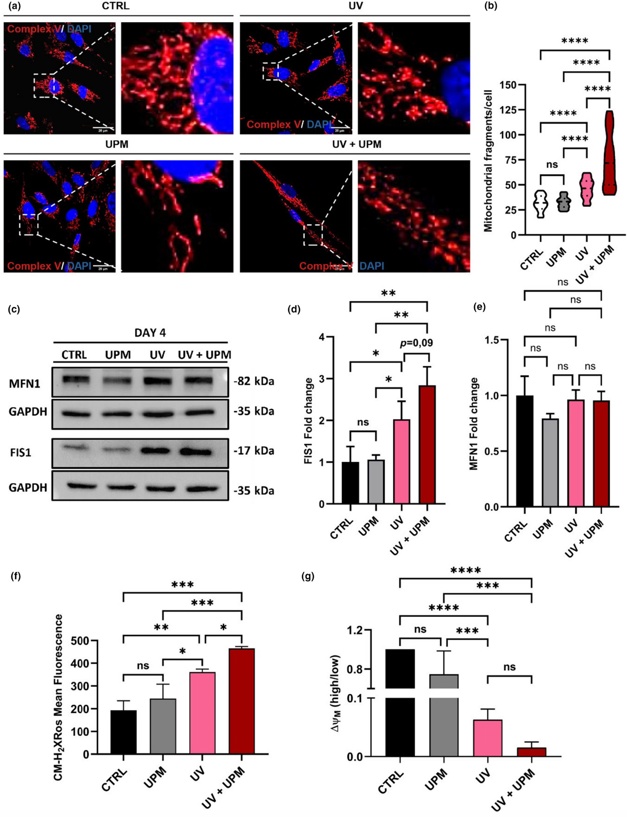
Synergistic Interplay of UV Radiation and Urban Particulate Matter in Senescence-Prone Human Dermal Fibroblasts
Skin aging is a complex process influenced by intrinsic factors and environmental stressors, including ultraviolet (UV) radiation and air pollution, among others. This study investigated the effects of UVA and UVB radiation, combined with urban particulate matter (UPM), on human dermal fibroblasts (HDF).
The results showed that treatment of HDF with a subcytotoxic dose of UVA/UVB led to a series of events, including mitochondrial dysfunction, increased ROS levels, and DNA damage. These effects are known to trigger either cellular senescence or cell death. Whereas UPM treatment in isolation did not affect proliferation or survival of HDF, of note, simultaneous UPM treatment of UV-irradiated cells selectively inhibited autophagic flux, thereby changing cell fate of a fraction of the cell population from senescence to apoptotic cell death.
These data highlight the synergistic effects of UV radiation and UPM on skin aging, emphasizing the need to consider these factors in assessing the impact of environmental stressors on human health and opening opportunities for developing comprehensive approaches to protect and preserve skin integrity in the face of growing environmental challenges.
Ask a Question
Write your own review