Rat Liver Sinusoidal Endothelial Cells
Cat.No.: CSC-C4183X
Species: Rat
Source: Liver
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat liver sinusoidal endothelial cells (LSECs) are rat endothelial cells isolated from the hepatic sinusoids. They form the lining of the liver's smallest blood vessels and are structurally characterized by unique fenestrations organized in sieve plates, the absence of a typical basement membrane, and a high endocytic capacity.
Rat LSECs involved in a variety of liver homeostasis mechanisms. They are a major scavenging population for blood-borne waste molecules and demonstrate a highly efficient clathrin-mediated endocytosis of soluble molecules through receptors such as stabilin-2, scavenger receptors and the macrophage mannose receptor. They are also immunologically active and have a role in regulation of hepatic fibrosis. LSECs have a gatekeeper function in preventing hepatic stellate cell (HSC) activation. In their differentiated state, they inhibit HSC activation and can actively drive reversal of activated HSCs to a quiescent state, predominantly via VEGF-stimulated nitric oxide (NO) production. However, following capillarization, or a loss of their fenestration and gain of a basement membrane, LSECs lose this ability and are permissive for HSC activation and fibrosis onset. They are often utilized in studies for in vitro modeling of liver physiology, toxicology, and disease biology. They have been widely used in studies of drug-induced liver injury, fibrosis, inflammation, and hepatocellular carcinoma.

L-ECM Hydrogels Slowed Down the Dedifferentiation of LSECs In Vitro
LSECs are key targets for MASLD, but their rapid dedifferentiation makes isolation and culture difficult. Wang's team used porcine liver-derived L-ECM to culture primary rat liver sinusoidal endothelial cells (LSECs) in hydrogels, aiming to explore the influence of L-ECM on liver cells and develop culture conditions that maintain their differentiated function in vitro.
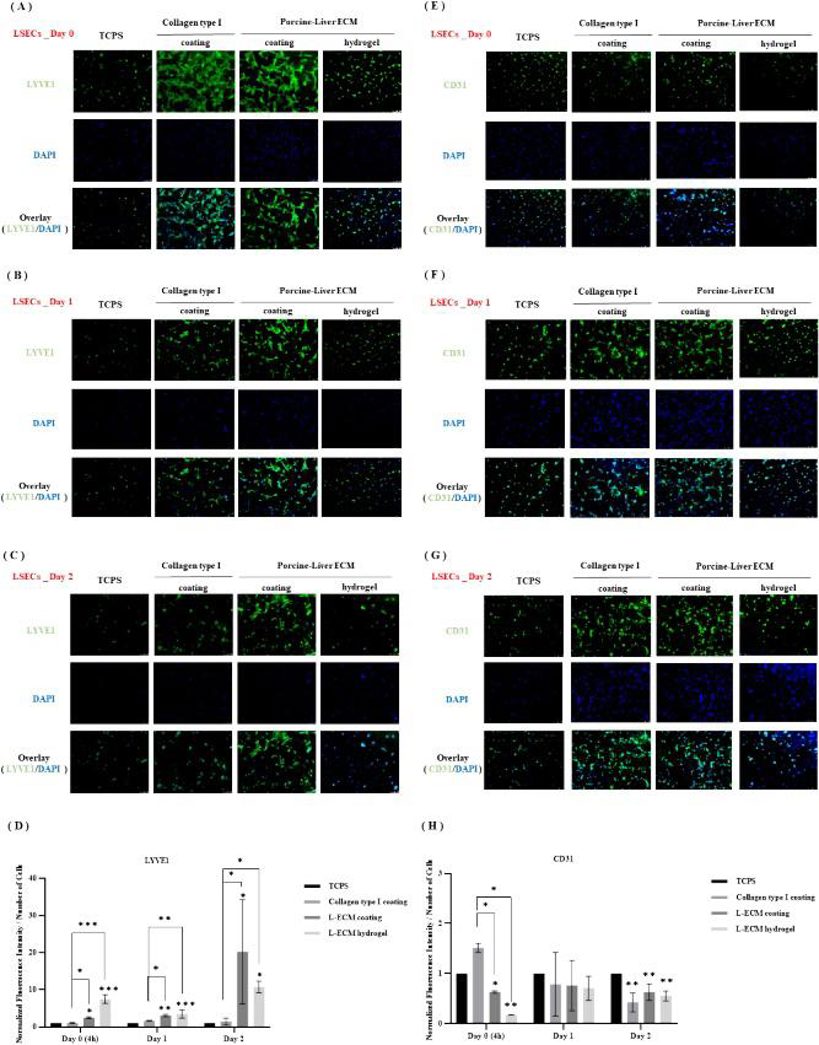
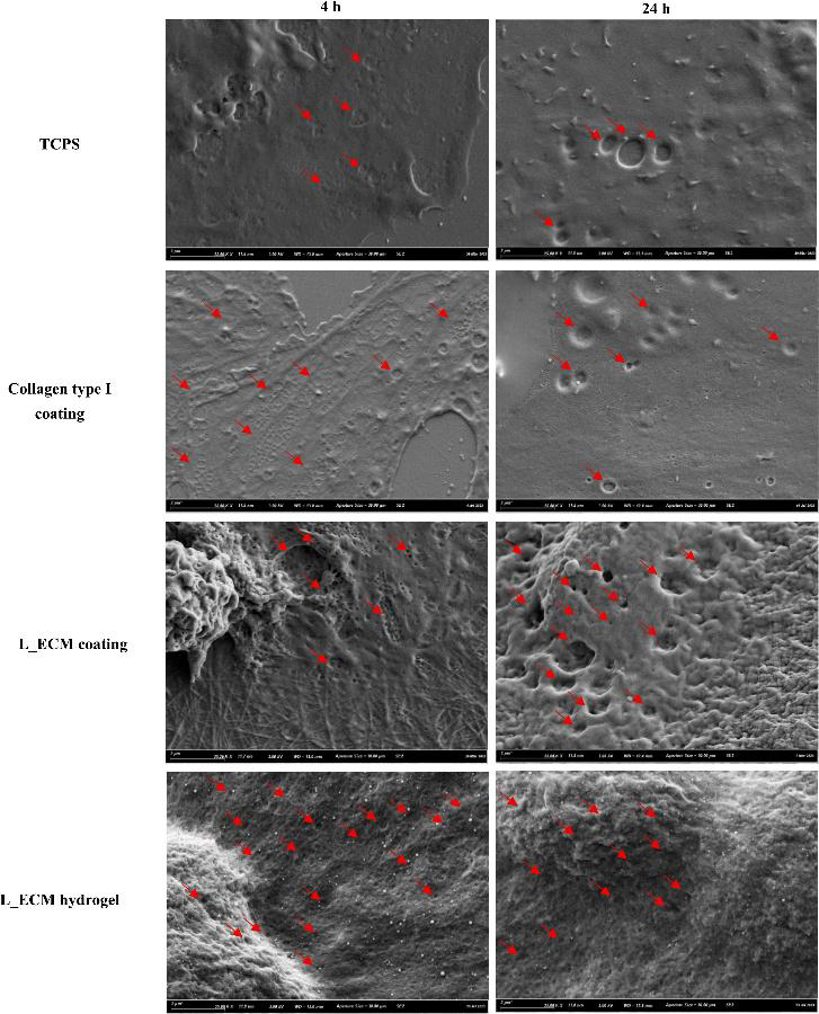
The expression of LSEC markers Stab2, Nos3, and LYVE1 decreased, while Mmp9 and CD31 increased over culture time, regardless of the substratum, though the rate varied (Fig. 1). On day 0 and day 2, Stab2 and Nos3 were higher in LSECs on L-ECM hydrogels than on TCPS or collagen. Mmp9 was significantly lower in LSECs on L-ECM coatings and hydrogels than on TCPS at day 2, with no significant differences at day 0 or day 1. LSECs on L-ECM hydrogels expressed more LYVE1 at all times compared to TCPS and collagen. CD31 expression was lower in LSECs on collagen and L-ECM coatings/hydrogels than on TCPS, especially at day 0 and day 2. Protein expression of CD31 was also lower in LSECs on L-ECM coatings/hydrogels than on TCPS and collagen at day 0, with no differences in LYVE1 and CD31 expression between L-ECM coatings and hydrogels (Fig. 1). LSECs on L-ECM coatings or hydrogels showed different morphology from those on TCPS or collagen. After 4 h, LSECs on TCPS had some fenestrae, while those on collagen and L-ECM had abundant fenestrae. These fenestrae gradually diminished or disappeared in all groups, but LSECs on L-ECM coatings or hydrogels still maintained some fenestrae, organized into sieve plates, characteristic of differentiated LSECs (Fig. 2).
ROS-induced Changes in LSEC Morphology
Reactive oxygen species (ROS) are prevalent in the liver during intoxication, infection, inflammation, and aging. Changes in liver sinusoidal endothelial cells (LSEC) are associated with various liver diseases. To deepen the knowledge about the LSEC and ROS interaction, Larissa et al. investigated the time-dependent and dose-dependent effects of H2O2 on rat LSEC morphology and functions in vitro.
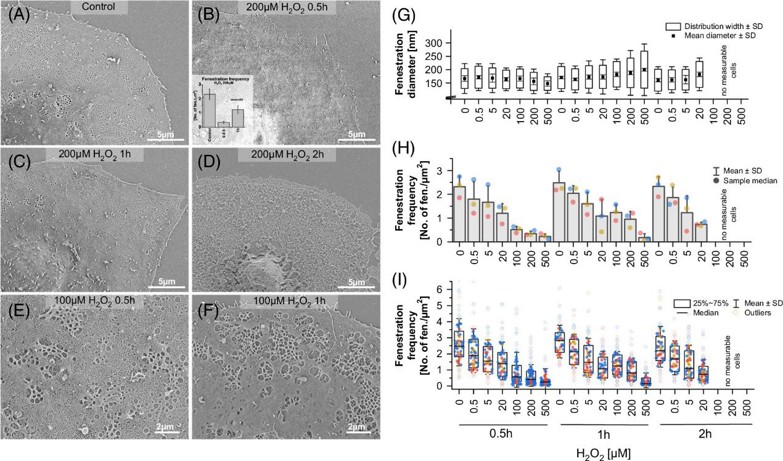
The effects of ROS on LSEC morphology were examined using microscopy. Scanning electron microscopy (SEM) was used to observe the detailed morphological structure (Figure 3A-F). Images were analyzed to calculate fenestration diameter, frequency, and porosity (Figure 3G-I). A significant, dose-dependent reduction in fenestration number was observed at all time points. Distorted sieve plates resembling defenestration centers (DFC) were seen at H2O2 concentrations above 5 µM (Figures 3B, C). Near complete defenestration was observed for 100-500 µM H2O2 after 0.5 hours, but fenestration numbers increased after 1 hour, though not to control levels (Figures 3B, H). Significant differences in fenestration frequency were noted between control and 500 µM H2O2 after 1 hour and between 30 minutes and 1 hour of 500 µM H2O2 treatment. The data show a heterogeneous cell population, with some cells remaining defenestrated and others regaining porosity (Figure 3I). For H2O2 concentrations above 100 µM, nearly no viable cells were observed after 2 hours, with most samples showing distorted/disrupted cell membranes, indicating necrosis (Figure 3D). This confirms previous data showing significant LDH release only after 2 hours of treatment with >100 µM H2O2. In samples treated with <20 µM H2O2, a dose-dependent loss of fenestrations was observed at all time points.
Ask a Question
Write your own review