Normal Human Epidermal Keratinocytes neonatal
Cat.No.: CSC-C4119X
Species: Human
Source: Epidermis; Skin
Cell Type: Keratinocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
HEKn are cryopreserved as primary cells. Cells are isolated from neonatal human foreskin and expanded once in culture vessels before cryopreservation.
HEKa are cryopreserved as primary cells. Cells are isolated from adult human skin and expanded once in culture vessels before cryopreservation.
HEK can be grown without serum, phenol red or antimicrobials when cultured in DermaLife medium.
HEK are extensively tested for quality and optimal performance.
Creative Bioarray guarantees performance and quality.
Normal Human Epidermal Keratinocytes Neonatal (NHEK-neonatal) are primary keratinocytes, isolated from the epidermis of human newborns and infants, usually from foreskin or abdominal skin tissue using enzymatic digestion. They are from neonatal sources and as such, have greater proliferative and differentiation potential than adult keratinocytes, making them useful for epidermal biology studies. NHEK-neonatal play important roles in epidermal homeostasis, including providing the physical barrier of the skin by forming keratin filaments and lipid-rich lamellar bodies and mediating innate immunity through antimicrobial peptides secretion (e.g. β-defensins). They are also key in wound healing processes by proliferating, migrating to the site of injury and differentiating to form a stratified epidermis.
Applications include their use in basic skin biology research, often to study epidermal differentiation mechanisms (e.g. upon calcium or vitamin D stimulation). They are used for toxicological testing in place of animal models to test for irritancy of cosmetics, pharmaceuticals, or industrial chemicals (per OECD guidelines). In tissue engineering, they are used as major components for the fabrication of 3D skin equivalents (e.g. with dermal fibroblasts) for burn wound grafts or in drug permeability studies. They can also be used to support research on skin diseases, e.g. atopic dermatitis, by recapitulating aspects of the pathological barrier dysfunction in vitro.
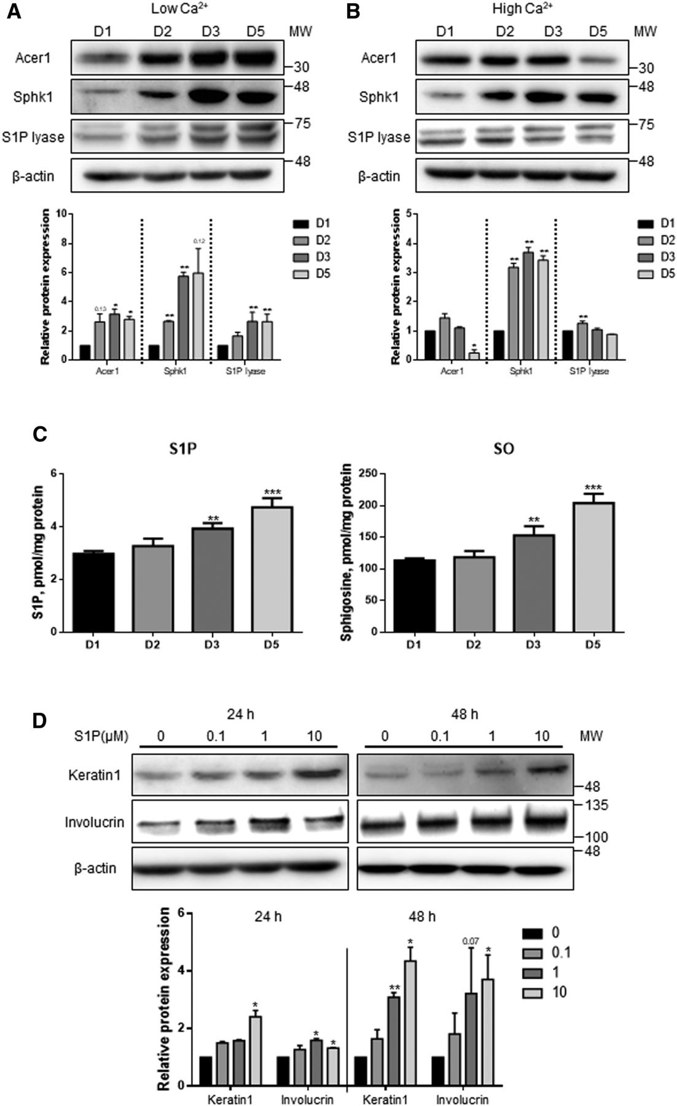
Human Epidermal Keratinocyte Differentiation is Associated with Increased S1P and Downregulated S1P Lyase
Sphingosine 1-phosphate (S1P) lyase is an enzyme that degrades S1P and has been suggested as a drug target for psoriasis treatment. As S1P promotes keratinocyte differentiation, Jeon et al. asked if manipulating S1P lyase would alter human neonatal epidermal keratinocyte (HEKn) cells proliferation and differentiation.
They inhibited S1P lyase in HEKn cells using an S1P lyase-specific inhibitor (SLI) and siRNA targeting S1P lyase 1 (SGPL1). HEKn cells were maintained in low Ca2+ (60 μM) or differentiated in high Ca2+ (2 mM) medium for 5 days. Western blot analysis revealed that expression of Acer1 and Sphk1, enzymes that produce S1P, were increased under low Ca2+ conditions. S1P lyase protein expression was increased over time under both low and high Ca2+ conditions (Fig. 1A). High Ca2+ downregulated Acer1 but upregulated Sphk1, with no change in S1P lyase expression (Fig. 1B). By using LC/MS/MS analysis they observed that the amount of S1P was increased over time under high Ca2+ conditions (Fig. 1C). Treatment with extracellular S1P enhanced protein levels of keratin 1 and involucrin, which are early and late markers of differentiation, respectively (Fig. 1D). Taken together, these data indicate that S1P metabolism is directly coupled to keratinocyte differentiation.
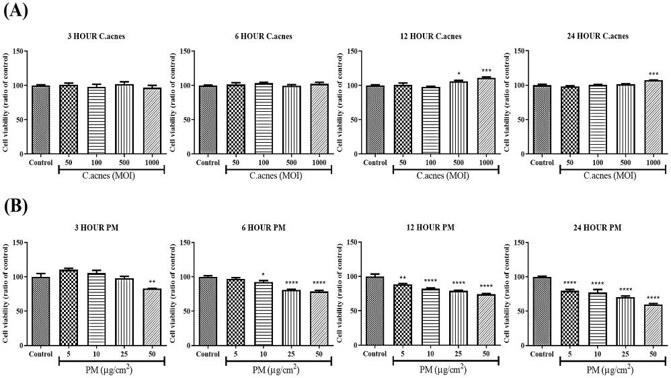
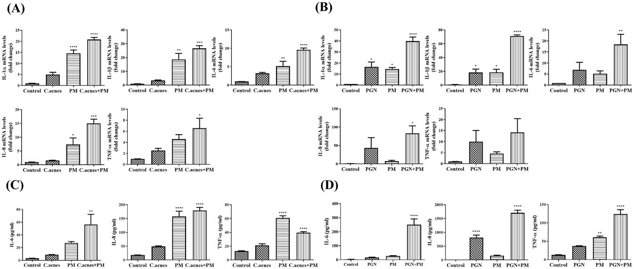
Effect of PM on Proinflammatory Cytokine Expression in C. acnes or PGN Treated HEKn Cells
Recent studies show that particulate matter (PM) can cause oxidative stress and inflammation linked to several skin conditions, but its effect on acne is unknown. Noh et al. investigated whether PM exposure worsens acne-like inflammation in HEKn cells induced by Cutibacterium acnes (C. acnes) and Staphylococcus aureus peptidoglycan (PGN).
Cytotoxicity tests showed no significant effect of C. acnes on HEKn cell viability (Fig. 2A), but PM caused significant cytotoxicity in a dose- and time-dependent manner (Fig. 2B). For further experiments, a PM concentration of 10 μg/cm² was used. HEKn cells were treated with PM and either heat-killed C. acnes or PGN. After 3 hours (mRNA) and 24 hours (protein), the expression of proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNF-α) was measured using qRT-PCR and ELISA. Results showed that PM significantly increased the expression of these cytokines in C. acnes- or PGN-treated cells (Fig. 3), indicating that PM can amplify acne-like inflammation.
Ask a Question
Write your own review


