
Human Tonsil Epithelial Cells (HTEpiC)
Cat.No.: CSC-7706W
Species: Human
Source: Tonsil
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Tonsil Epithelial Cells (HTEpiC) are primary, non‑immortalized epithelial cells that have been isolated from palatine tonsils obtained from healthy donors and cryopreserved at passage 1. When seeded onto poly‑L‑lysine‑coated (2 µg cm⁻²) culture vessels and maintained in the media with growth factors Tonsil Epithelial Cell Medium (TEpiCM), HTEpiC will adhere as a homogeneous squamous monolayer, exhibit characteristic cytokeratin profiles (CK5, CK14, CK8/18, CK19) and will maintain the heterogeneous differentiation as seen in vivo.
HTEpiC express both α2,3‑ and α2,6‑linked sialic acid receptors, which makes them permissive to a wide range of respiratory pathogens including influenza A subtypes, Epstein‑Barr virus, human papillomavirus and adenoviruses. Infection of HTEpiC results in a robust release of IL‑6, IL‑8 and TNF‑α, making this line a useful model for studying epithelial innate immunity and cytokine‑mediated inflammation. As the cells retain a physiologically relevant barrier and receptor repertoire, they are also broadly used for antiviral drug screening, high‑throughput toxicity testing and mechanistic studies of bacterial‑host interactions, such as Staphylococcus aureus and Group A Streptococcus.
Effect of Carvacrol on Cell Viability and Morphological Changes of Human Tonsil Epithelial Cells and Inflammation Model
Pharyngitis or sore throat can be caused by viruses, bacteria, or non-infectious factors. Wijesundara et al. report the use of an in vitro model of streptococcal pharyngitis to test the anti-inflammatory effect of carvacrol. Human tonsil epithelial cells (HTonEpiCs) were pre-stimulated with Streptococcus pyogenes cell wall antigens and subsequently exposed to carvacrol.
They had previously established that carvacrol (3.9-250 µg/mL) is not cytotoxic to HTonEpiCs using the MTS assay. To validate these findings, they incubated HTonEpiCs with carvacrol (8-250 µg/mL) or control (DMSO, nimesulide) for 24 h and determined cell viability by flow cytometry. They found that carvacrol maintained viability above 80% (Fig. 1A, B) and there was no significant morphological difference when compared to DMSO-treated cells (Fig. 1C). Nimesulide, a known NSAID, was also not cytotoxic. Thus, the concentrations of carvacrol used in the subsequent experiments were 16.1, 31.2, 62.5, 125, and 250 µg/mL (in DMSO) and 4 µg/mL nimesulide. After LTA + PGN stimulation for 4 h, HTonEpiCs showed changes in cellular morphology, such as irregular shape, smaller size, and lower cell density (Fig. 2). These changes were more severe after 24 h.


Influenza Virus Susceptibility and Growth Kinetics in Differentiated HTEC Cultures
The tonsils are known sites of replication for various respiratory viruses, including Epstein-Barr virus and influenza. Human tonsil epithelial cells (HTECs) are diverse and actively differentiating. Perry et al. examined differentiated human tonsil epithelial cells (HTECs) for their susceptibility to influenza viruses.
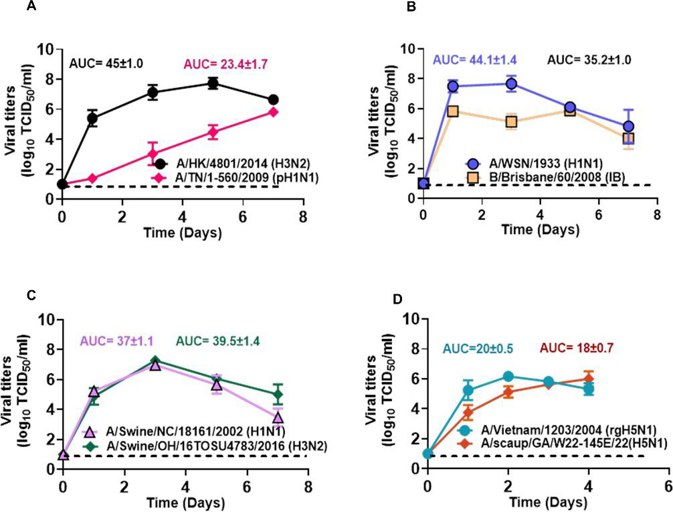
To see if well-differentiated HTECs can support the replication of human, swine, and avian influenza viruses, they examined the growth of eight strains (Fig. 3). All viruses replicated in HTECs, reaching peak titers of about 6 × 106 TCID50/mL. This shows that HTECs support diverse influenza viral strains. The most delayed replication was seen in A/TN/1-560/2009 (pH1N1; Fig. 3A). The other seven strains, including A/HK/4801/2014 (H3N2), B/Brisbane/60/2008 (IB), A/WSN/1933 (H1N1), A/swine/OH/16TOSU4783/2016 (H3N2), A/swine/NC/18161/2002 (H1N1), A/Vietnam/1203/2004 (rgH5N1), and A/scaup/GA/W22-145E/2022 (H5N1), replicated quickly and reached measurable titers by 1 dpi. B/Brisbane/60/2008 (IB) and A/WSN/1933 (H1N1) peaked by 1 dpi (Fig. 3B). A/HK/4801/2014 (H3N2) peaked at 5 dpi. A/TN/1-560/2009 (pH1N1) replicated slowly and did not peak within 7 days (Fig. 3A). Both swine viruses peaked at 3 dpi (Fig. 3C). HPAI A(H5N1) viruses replicated efficiently, causing significant cytopathic effects by 4-5 dpi (Fig. 3D). Overall, HTECs support the replication of swine, avian, and human influenza viruses, making them key sites for viral replication in the respiratory tract.
Ask a Question
Write your own review

