Human Perineurial Cells (HPC)
Cat.No.: CSC-7737W
Species: Human
Source: Perineurium
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human perineurial cells (HPC) are isolated from human spinal nerves. These cells execute substantial cytoplasmic endocytosis while containing numerous actin and vimentin filaments that support the structural integrity of nerve sheaths.
Perineurial cells serve as protective barriers which guard nerve fibers against physical damage and harmful substances. These cells function to transmit neural signals and managing nerve regeneration processes. For example, scientific findings reveal that perineurial cells promote nerve fiber regeneration by releasing structural matrix components including type I collagen and fibronectin. Perineurial cells play vital roles in various disease states. For instance, nerve sheath tumors including neurofibromatosis and schwannomas exhibit abnormal growth of perineurial cells. Furthermore, regeneration potential exists in perineurial cells which become active during nerve injury repair. Current knowledge does not fully explain how perineurial cells operate following nerve injury.
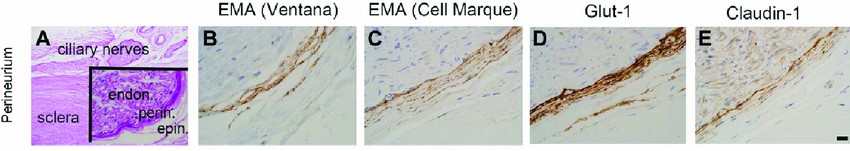 Fig. 1. Immunophenotype of perineurial cells (Gilbert A, Chévez-Barrios P, et al., 2018).
Fig. 1. Immunophenotype of perineurial cells (Gilbert A, Chévez-Barrios P, et al., 2018).
Effect of EGFR Inhibition on Cell–Cell Junctions of HPNCs
The perineurium plays a vital role in the blood–nerve barrier, crucial for managing neurosensory disturbances resulting from oral surgeries and chemotherapy. Composed of cell–cell junction proteins, its function in regulating permeability is not well understood. Hiraoka et al. examined the expression of JCAD and EGFR in the perineurium of the human inferior alveolar nerve and uses cultured human perineurial cells (HPNCs) to understand these proteins' roles.
They analyzed the effect of EGFR inhibition on HPNCs to investigate the role of EGFR in the regulation of perineurial cell–cell junctions. Inhibition of EGFR phosphorylation by AG1478 changed the spindle-shaped fibroblastic morphology in control cells to a flattened morphology (Fig. 1A). Although EGFR inhibition did not change the total cell number or cell surface area, the degree of cell elongation was significantly changed in a dose-dependent manner (Fig. 1B) Notably, although scattered dot-like expression of JCAD at cell–cell contacts was prominent in control cells, the cells with long and linear expression of JCAD at cell–cell contacts appeared among AG1478-treated cells. To confirm this change, we calculated the ratio of JCAD-positive regions occupying cell–cell contacts per single cell (Fig. 2A). EGFR inhibition significantly increased the ratio of JCAD-positive cell–cell contacts of AG1478-treated cells compared with control cells (average ratio was 12.9% in control cells and 29.2% in 10-μM AG1478-treated cells).
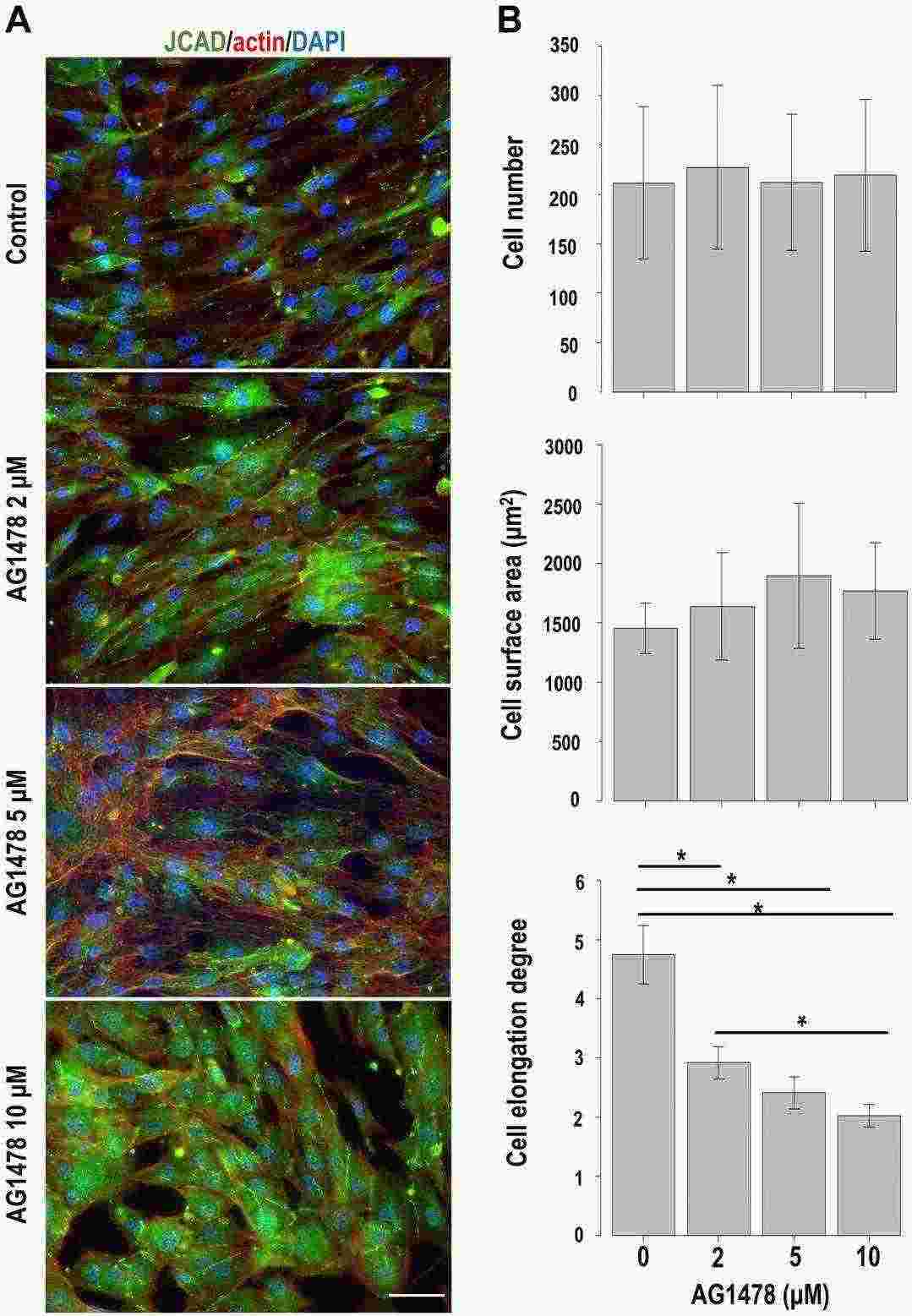 Fig. 1. Changes induced by EGFR inhibition in cultured human perineurial cells (Hiraoka Y, Matsumura M, et al., 2023).
Fig. 1. Changes induced by EGFR inhibition in cultured human perineurial cells (Hiraoka Y, Matsumura M, et al., 2023).
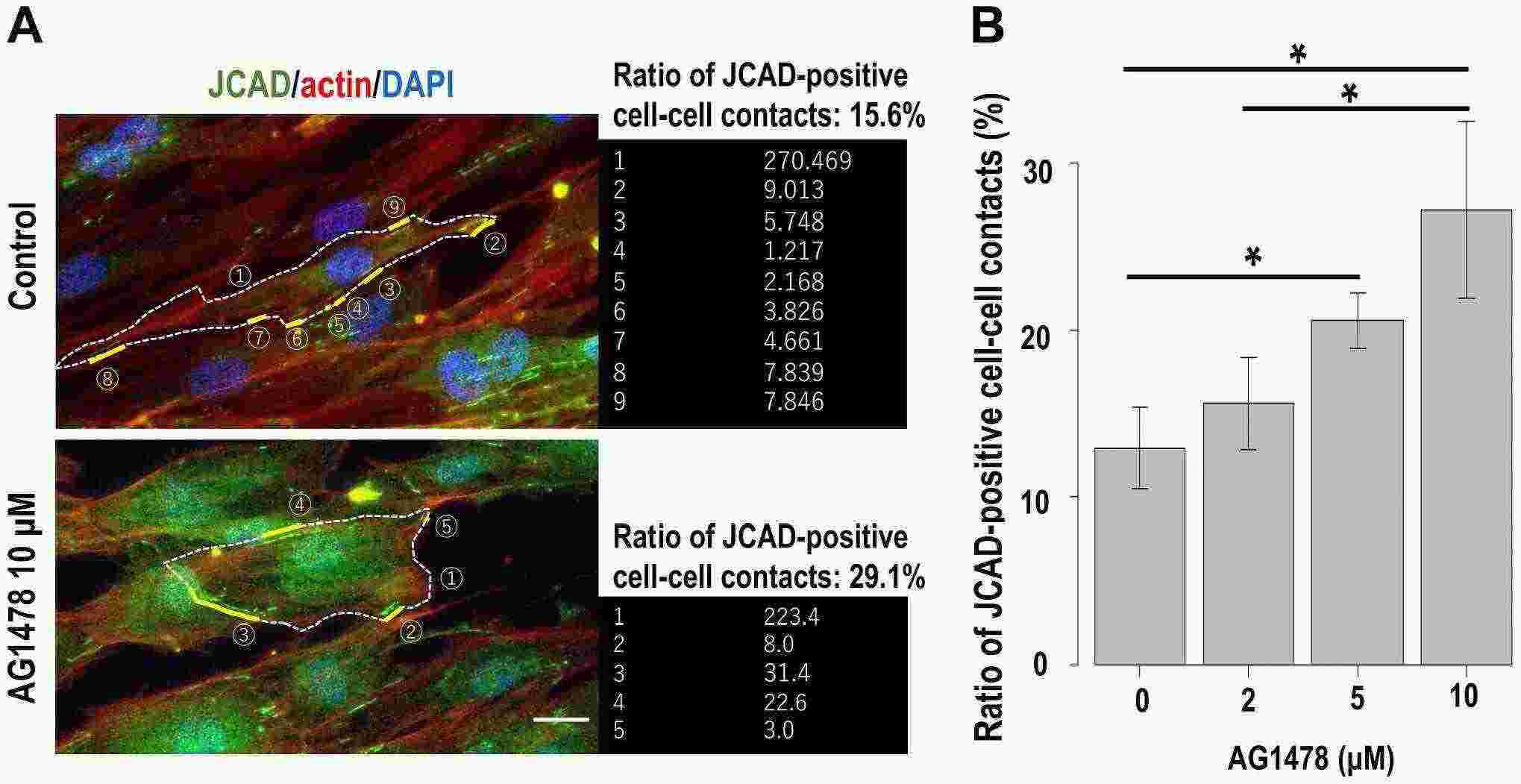 Fig. 2. Effect of EGFR inhibition on cultured human perineurial cells. (A) Details of the calculation method of the ratio of JCAD (green)-positive cell–cell contacts (Hiraoka Y, Matsumura M, et al., 2023).
Fig. 2. Effect of EGFR inhibition on cultured human perineurial cells. (A) Details of the calculation method of the ratio of JCAD (green)-positive cell–cell contacts (Hiraoka Y, Matsumura M, et al., 2023).
Claudin-1 Redistributes from Cell Membrane/Cytoplasm to Nucleus During VZV Infection and Is Not Essential for Virus Entry
In temporal arteries (TAs) from patients with giant cell arteritis, varicella zoster virus (VZV) is seen in perineurial cells that surround adventitial nerve bundles and form the peripheral nerve-extrafascicular tissue barrier (perineurium). Blackmon et al. examined VZV's presence in the temporal arteries of GCA patients, where it disrupts perineurial cells. Using mock- and VZV-infected primary human perineurial cells, the research assesses changes in cell adhesion proteins, influenced by interleukin-6.
At 3 DPI, claudin-1 was located in the membrane/cytoplasm of all mock-infected qHPNCs, confirming their identity as perineurial cells (Fig. 3B, left panel, green). In VZV-infected cells (red), claudin-1 (green) moved to the nucleus but stayed in the cytoplasm of uninfected cells (Fig. 3B, right panel, arrows). RT-qPCR showed no significant difference in claudin-1 levels between mock- and VZV-infected cells (1.00 ± 0.00 versus 0.77 ± 0.11, fold difference ± SD, n = 5). Testing if claudin-1 affected VZV entry, qHPNCs were pretreated with an isotype control or anti-claudin-1 antibody before VZV infection; FACS showed similar VZV gE expression in both groups (Fig. 3C; 79 ± 0.45 versus 81 ± 0.93, P < .17, percent expression ± SD, n = 4).
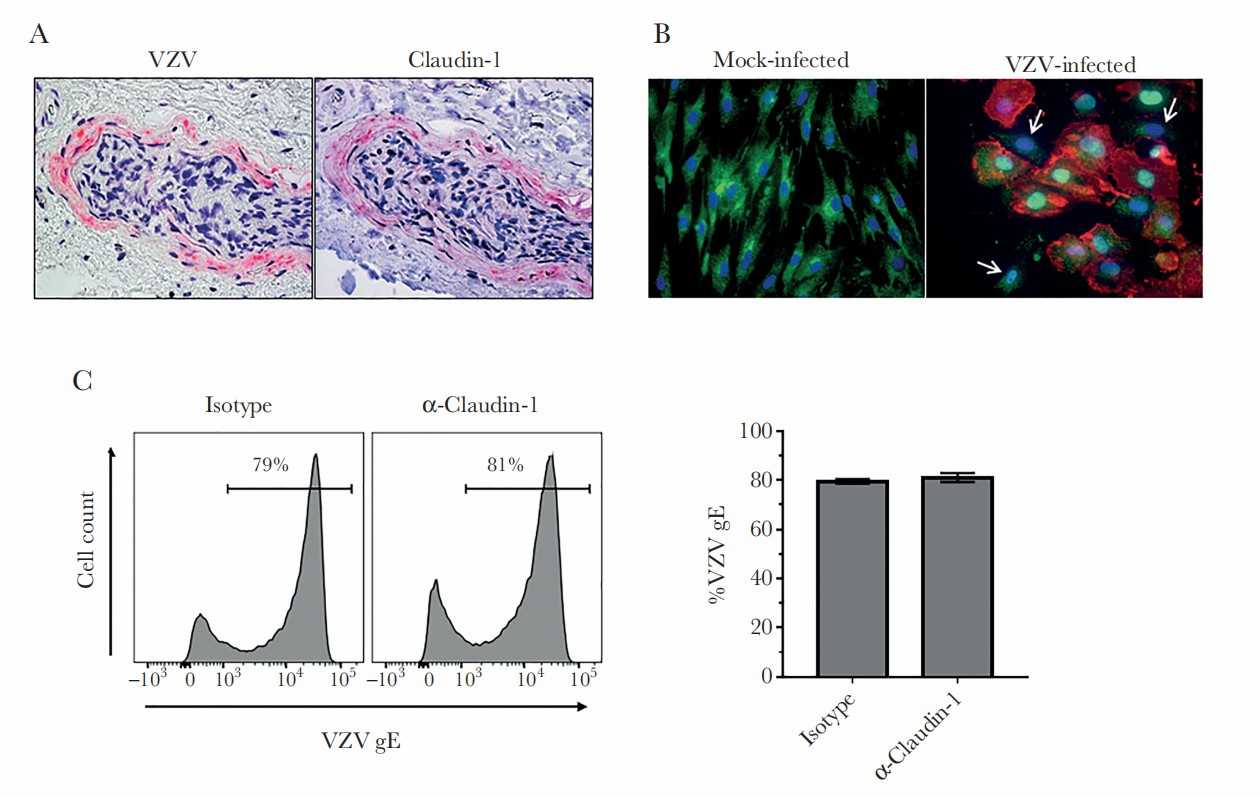 Fig. 3. Varicella zoster virus (VZV) in perineurial cells expressing claudin-1 (Blackmon A M, Como C N, et al., 2019).
Fig. 3. Varicella zoster virus (VZV) in perineurial cells expressing claudin-1 (Blackmon A M, Como C N, et al., 2019).
Ask a Question
Write your own review