Human Meningeal Cells (HMC)
Cat.No.: CSC-7758W
Species: Human
Source: Brain
Cell Type: Meningeal Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Meningeal Cells are stromal cells derived from the meninges, the three-layered membrane (the dura mater, arachnoid, and pia mater) that encases the brain and spinal cord. Meningeal cells can be isolated from either the dura mater or the leptomeninges (arachnoid and pia) with the majority of research utilizing cells derived from the latter source. Meningeal cells secrete ECM proteins such as collagen IV and laminin as well as neurotrophic factors such as BDNF and GDNF to maintain the blood-brain barrier, cerebrospinal fluid homeostasis and regulate neural stem cell niches.
Meningeal cells have been used in research to study normal neurodevelopmental processes, CNS infections, and leukemia metastasis to the CNS. Meningeal cells can affect neuronal differentiation in a paracrine manner through the secretion of factors such as RETN/REELIN and have been used as a tool to understand cortical layering diseases. Primary cells or immortalized lines (such as HMC3) are commercially available and are generally cultured in DMEM/F12 with the addition of bFGF. Meningeal cells have also been used recently to develop more advanced 3D organoid co-culture systems to recapitulate brain microenvironment interactions, with a focus on studying cancer chemoresistance and neuroinflammatory diseases.
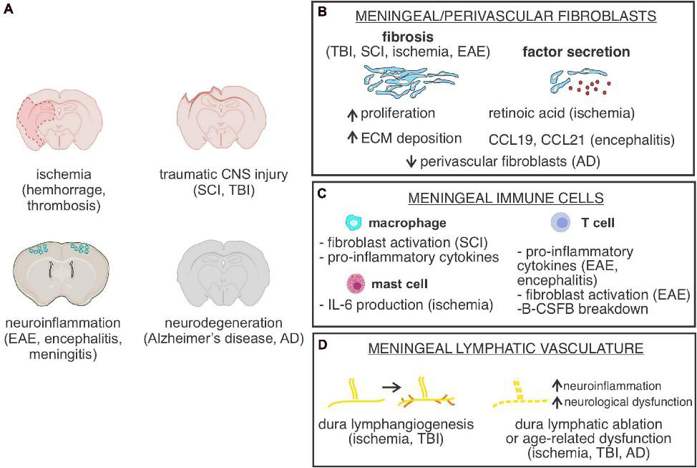
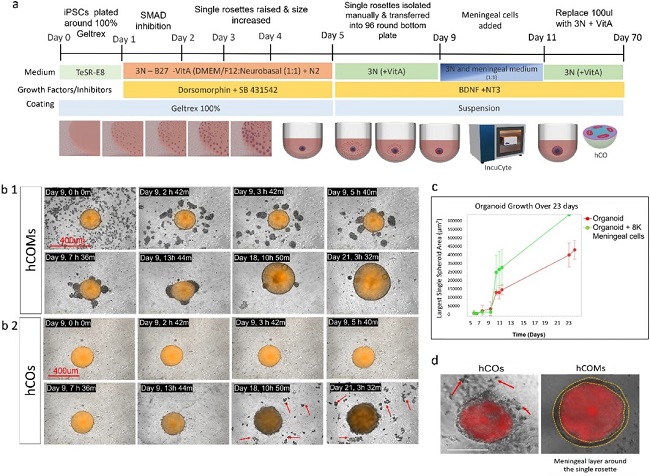
Presence of Meningeal Cells Significantly Enhanced the Morphology and Phenotype of Cortical Organoids
Current human cortical organoid (hCO) models lack essential non-neural elements, such as meningeal layers required for physiologically-relevant corticogenesis, precluding proper modeling of brain development or neurodevelopmental disorders (NDDs). Here, Jailian et al. sought to create a new 3D co-culture system of hCO with meningeal cells isolated from early developmental stages.
Using an optimized co-culture system with addition of 8,000 meningeal cells to each single rosette at day 9 in vitro, they found that meningeal cells attached within 24h and enwrapped rosettes (Fig.1b1) giving rise to hCOMs with a significantly improved spherical morphology compared to controls (Fig. 1b2 and d). In real time, hCOMs also released less debris (Fig. 1b2 red arrows) and 1.5-fold more expansion compared to controls over 21 days (Fig. 1c), a result validated across 3 cell lines (CC1, PCDH19 and CHD2). Collectively, these findings demonstrate that our novel co-culture system from human iPSCs and meningeal cells together can efficiently improve the morphology and phenotype of cortical organoids and increase their growth in a defined time course.
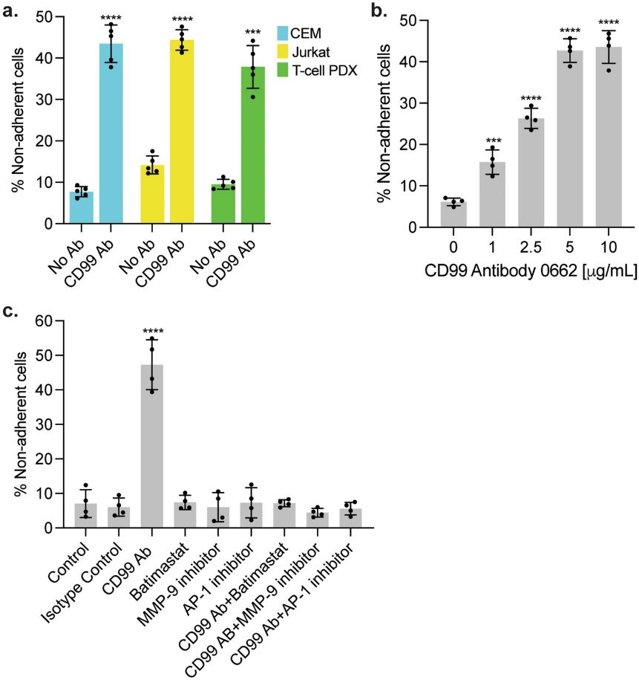
CD99 Antibody Disrupts Leukemia-Meningeal Cell Adhesion
Despite high cure rates for acute lymphoblastic leukemia (ALL), CNS relapse remains a major therapeutic challenge due to chemoresistance mediated by leukemia-meningeal cell adhesion. Previous studies identified CD99 as a potential regulator of these interactions. Ebadi et al. investigated how CD99 antibody disrupts leukemia-meningeal adhesion and reverses chemoresistance, providing a novel therapeutic strategy for CNS relapse.
To assess CD99 antibody's impact on leukemia-meningeal adhesion, Jurkat/CEM T-ALL cells and primary T-ALL cells were co-cultured with human meningeal cells ± CD99 antibody (clone 0662). After 24h, flow cytometry with counting beads was performed to enumerate non-adherent leukemia cells after antibody treatment. The number of non-adherent leukemia cells was significantly increased with antibody treatment indicating disruption of adhesion (Fig. 2a). Pre-treatment of either cell type with antibody similarly led to an impairment of leukemia-meningeal adhesion. Dose response experiments indicated that the number of non-adherent cells was directly proportional to antibody concentration (Fig. 2b).
Ask a Question
Write your own review