Mouse Ventricular Cardiomyocytes
Cat.No.: CSC-C5301S
Species: Mouse
Source: Heart
Cell Type: Cardiomyocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse ventricular cardiomyocytes from Creative Bioarray are isolated from the mouse heart tissue. The method we use to isolate mouse ventricular cardiomyocytes was developed based on a combination of established and our proprietary methods. The mouse ventricular cardiomyocytes are characterized by immunofluorescence with antibodies specific to myosin heavy chain. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse ventricular myocytes are commonly obtained by extracting ventricular tissue from mouse hearts at various developmental stages, such as embryonic, neonatal, and adult phases. Ventricular myocytes serve as the heart's main force generators which drive the movement of blood through their contractile actions. These cells activate contractions through their response to both calcium release and electrophysiological signals. The electrophysiological properties of ventricular myocytes which include spontaneous calcium release and action potentials alongside calcium waves (DADs) position them as perfect models to analyze arrhythmias and cardiomyopathy.
Scientists analyze ventricular myocytes to explore molecular pathways that lead to cardiac hypertrophy, arrhythmias and myocardial infarction. For example, research into Ang II-induced cardiac hypertrophy has clarified how HDAC2 and JAK2 signaling pathways function. The stable electrophysiological properties of ventricular myocytes make them a preferred model for drug evaluation of cardiac function in research. For instance, puerarin prevents the development of cardiac hypertrophy induced by Ang II through its action on the ERK1/2, p38, and NF-κB signaling pathways. Moreover, scientists study cardiac repair and regeneration methods through the use of ventricular myocytes to transform induced pluripotent stem cells into cells similar to ventricular myocytes for artificial heart tissue creation.
OxLDL-Induced Reduction of Cardiomyocyte Cell-Shortening Requires PCSK9
PCSK9 functions in liver LDL metabolism which plays a vital role in maintaining cardiovascular health. The fact that PCSK9 shows expression in cardiomyocytes and is regulated by oxidized LDL (oxLDL) demonstrates possible non-hepatic roles in cardiac cell function.
To investigate whether endogenously expressed PCSK9 alters cell shortening of adult ventricular cardiomyocytes, Wolf et al. isolated adult ventricular cardiomyocytes from PCSK9?/?- as well as PCSK9+/+-mice. Comparison between both strains revealed no obvious difference (Fig. 1). Cardiomyocytes from both strains were cultivated under serum-free conditions and also incubated for 24 h with oxLDL (5 μM). Thereafter function was measured by load-free cell shortening. The cardiomyocytes showed reduced relative cell shortening after oxLDL (5 μM) incubation (Fig. 1a). as well as contraction (Fig. 1b) and relaxation velocities (Fig. 1b). PCS9+/+-cardiomyocytes showed no significant change under the same conditions when isolated from PCSK9?/?-mice (Fig. 1a–c). The absence of PCSK9 resulted in faster contraction and relaxation speeds in cardiomyocytes treated with oxLDL (Fig. 1b and c).
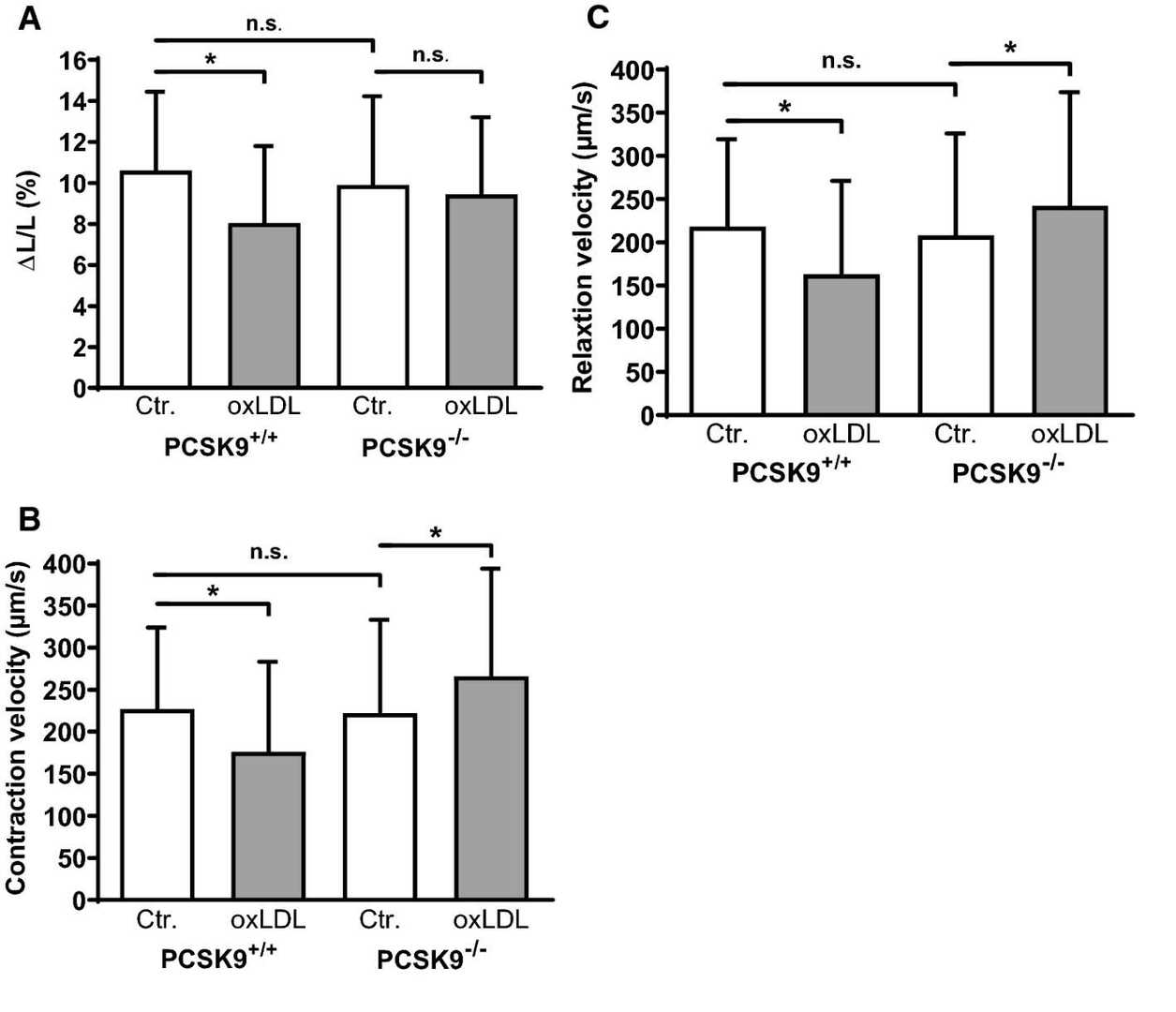 Fig. 1. Effect of oxLDL on load-free cell shortening of isolated cardiomyocytes derived from PCSK9?/?- and PCSK9+/+-mice (Wolf A, Schreckenberg R, et al., 2020).
Fig. 1. Effect of oxLDL on load-free cell shortening of isolated cardiomyocytes derived from PCSK9?/?- and PCSK9+/+-mice (Wolf A, Schreckenberg R, et al., 2020).
Mepivacaine Reduces Calcium Transients in Isolated Murine Ventricular Cardiomyocytes
Mepivacaine impacts cardiac function, causing arrhythmias by altering Na+ current in heart cells. Given its observed myocardial depression, Mosqueira et al. aim to investigate how mepivacaine influences Ca2+ transients in isolated mouse ventricular cardiomyocytes to better understand its negative inotropic effect.
To determine the IC50 of mepivacaine, the peak area from Ca2+ transients was analyzed across various concentrations: 0, 10, 20, 40, 50, 75, and 100 μM (Fig. 2a and b). The IC50 was estimated at 50.85 ± 6.79 μM, so 50 μM was used for subsequent testing. At this concentration, mepivacaine significantly reduced Ca2+ transient aspects, including baseline (control: 356.8 ± 42.12 nM; mepivacaine: 132.2 ± 14.55 nM, p < 0.05; Fig. 3b), peak area (control: 401.7 ± 63.09 nMs; mepivacaine: 72.14 ± 10.46 nMs, p < 0.05; Fig. 3c), and D50 (control: 457.1 ± 47.16 ms; mepivacaine: 284.5 ± 22.71 ms, p < 0.05; Fig. 3d), while the total duration remained unchanged (p > 0.05; Fig. 3e). Reduction in peak (Fig. 4a), slope (Fig. 4b), and time to peak (Fig. 4c) were also observed. However, re-uptake measures showed no significant effect on tau and time to uptake.
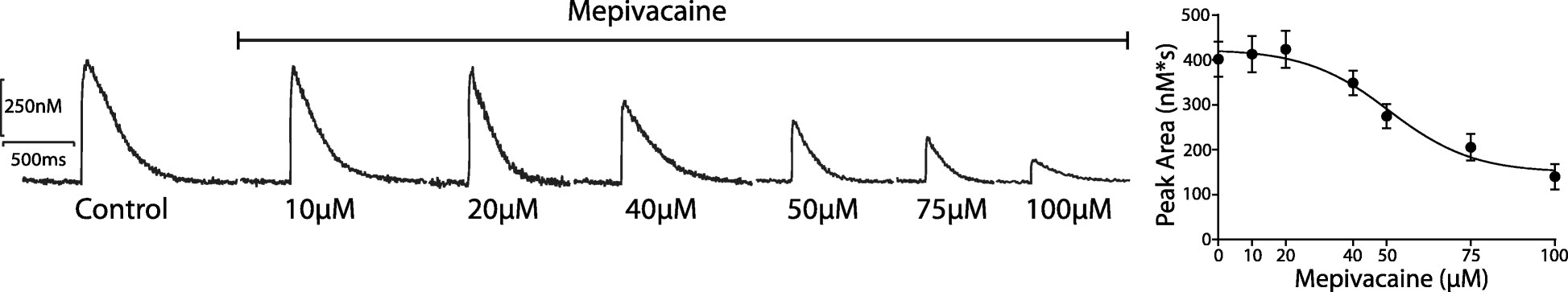 Fig. 2. Representative Ca2+ transients' traces to the dose-response effect of mepivacaine at 0, 10, 20, 40, 50, 75 and 100 μM (Mosqueira M, Aykut G, et al., 2020).
Fig. 2. Representative Ca2+ transients' traces to the dose-response effect of mepivacaine at 0, 10, 20, 40, 50, 75 and 100 μM (Mosqueira M, Aykut G, et al., 2020).
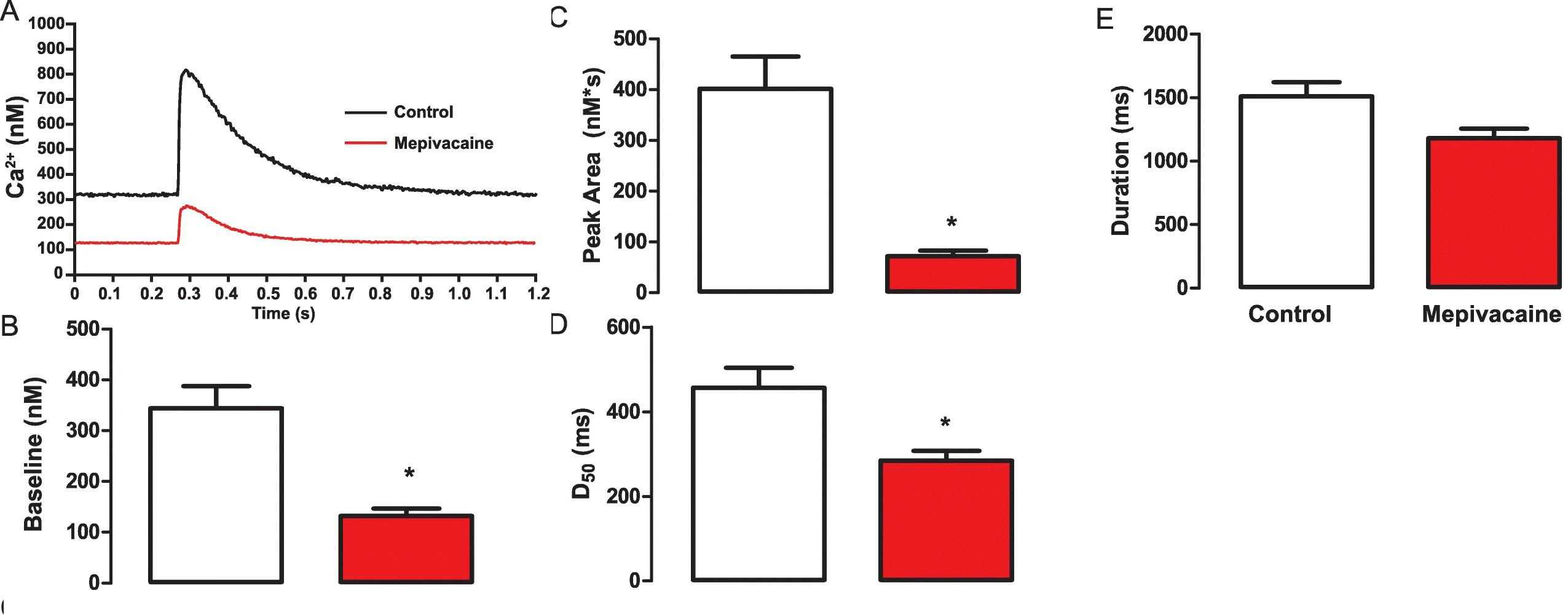 Fig. 3. Representative Ca2+ transients trace demonstrating the inhibitory effect of mepivacaine at half maximal inhibitory concentration (IC50) on in isolated mouse ventricular cardiomyocytes (Mosqueira M, Aykut G, et al., 2020).
Fig. 3. Representative Ca2+ transients trace demonstrating the inhibitory effect of mepivacaine at half maximal inhibitory concentration (IC50) on in isolated mouse ventricular cardiomyocytes (Mosqueira M, Aykut G, et al., 2020).
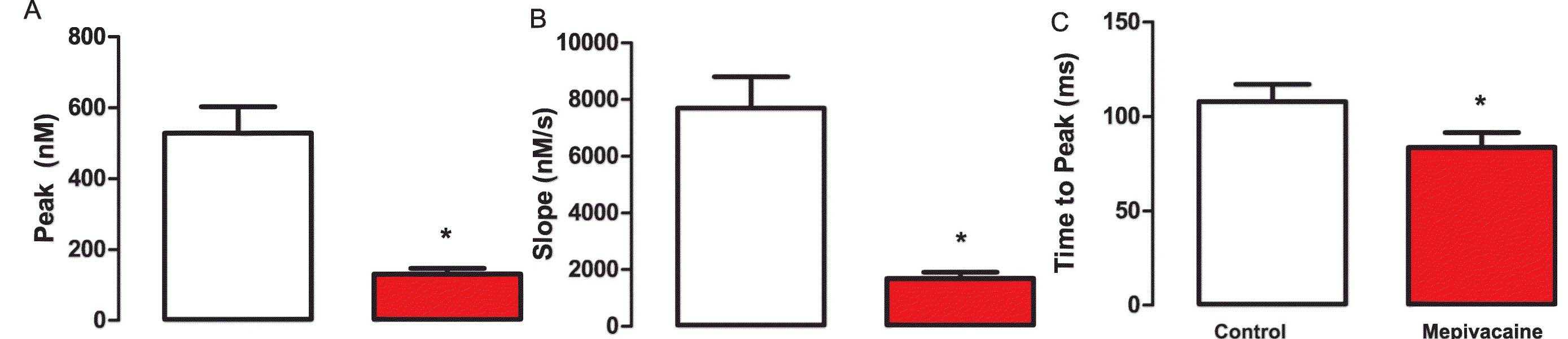 Fig. 4. Summarized data for the effect of mepivacaine at IC50 on Ca2+ release's biophysical parameters obtained from Ca2+ transients (Mosqueira M, Aykut G, et al., 2020).
Fig. 4. Summarized data for the effect of mepivacaine at IC50 on Ca2+ release's biophysical parameters obtained from Ca2+ transients (Mosqueira M, Aykut G, et al., 2020).
Ask a Question
Write your own review