Mouse Skeletal Muscle Fibroblasts
Cat.No.: CSC-C5388S
Species: Mouse
Source: Skeletal Muscle
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse skeletal muscle fibroblasts from Creative Bioarray are isolated from the mouse leg muscle tissue. The method we use to isolate mouse skeletal muscle fibroblasts was developed based on a combination of established and our proprietary methods. The mouse skeletal muscle fibroblasts are characterized by immunofluorescence with antibodies specific to vimentin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse skeletal muscle fibroblasts are cells found within the connective tissue of the muscle, most notably within the endomysium, or the connective tissue that individually wraps the muscle fibres. Fibroblasts within the skeletal muscle provide an important role in supporting the structural integrity of muscle and synthesise the ECM components (ECM) such as collagen and fibronectin that control the architecture of the tissue. They are also responsible for regulating growth signalling of the muscle through secretion of FGF (fibroblast growth factor), and play a role in muscle regeneration, often with satellite cells, to repair damage to the tissue after injury.
In research, skeletal muscle fibroblasts serve as valuable tools across multiple disciplines. They can be used in tissue engineering to produce in vitro skeletal muscle models to study the regeneration of muscle fibres, and they are also relevant in the study of ECM fibrotic pathologies, such as muscular fibrosis and dystrophies, due to their role in ECM remodelling. These cells have also been derived as precursors to fibroblast derived stem cells, which can be potentially useful in regenerative medicine applications.
D-Gal-Induced Skeletal Muscle Fibrosis Characteristics
Sarcopenia is characterized by muscle fiber loss and excessive fibrosis. Cellular senescence plays a vital role in its unclear pathogenesis. D-galactose (D-gal) can lead to senescence and fibrosis. However, the key regulator of this process remains unknown. Shi's team demonstrated the mechanism of D-gal-induced senescence and fibrosis in fibroblasts and provided a potential target for therapeutic intervention to inhibit the aging process in skeletal muscle.
D-gal has been found to cause cellular senescence characterized by mitochondrial damage and impaired energy metabolism. In NOR-10 fibroblasts, D-gal treatment increased the levels of senescence-related markers p16 and p53, which were confirmed by SA-β-gal staining (Fig. 1A, B). An aging model was successfully established in mouse skeletal muscle fibroblasts. Western blot analysis indicated an increase in the expression of fibrosis markers (α-SMA, COL-1) after D-gal-induced senescence (Fig. 1C). D-gal has been extensively used for inducing skeletal muscle aging. After 8 weeks of D-gal injection, body weight was determined, and muscle strength was measured. In addition, lower limb muscle weights (gastrocnemius, tibialis anterior, quadriceps) were measured to compare the D-gal group with the control group (Fig. 2A). The D-gal group showed significantly decreased muscle strength and relatively low lower limb muscle weights. HE and Masson histological analyses showed decreased fiber cross-sectional area and increased extracellular matrix (ECM) in the D-gal group (Fig. 2B and C).
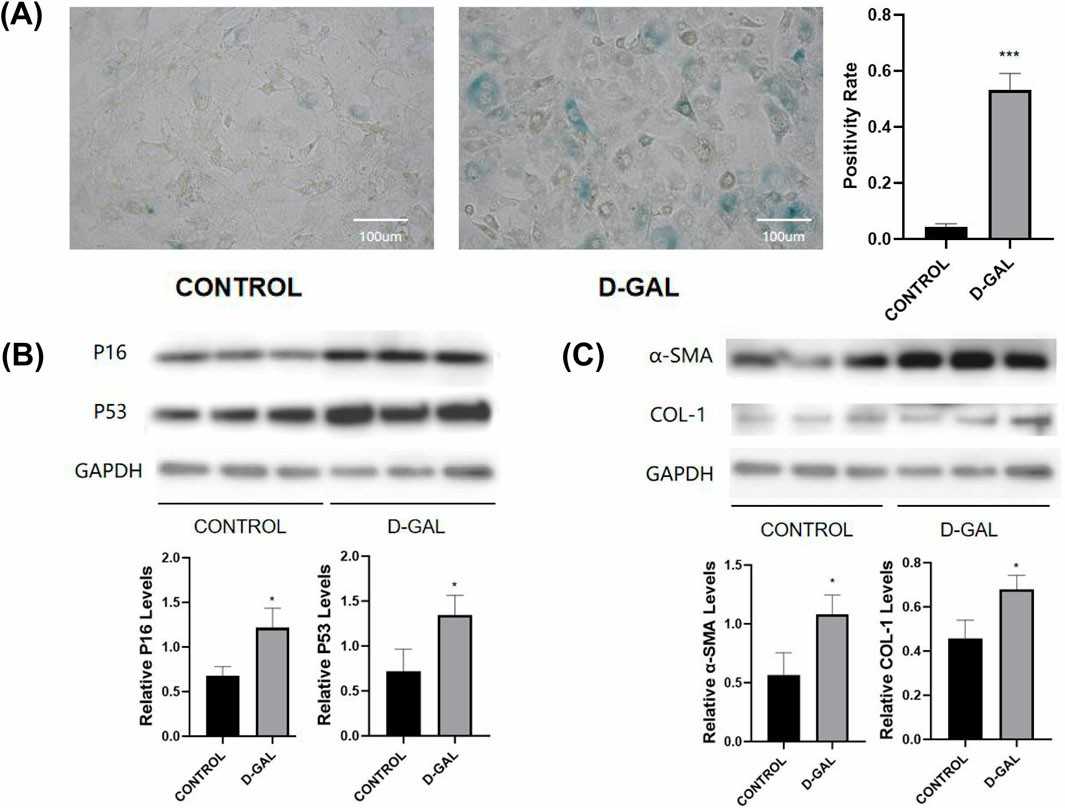 Fig. 1. D-Gal-Induced Senescence and Fibrosis in Fibroblasts (Shi L, Ding Z, et al., 2025).
Fig. 1. D-Gal-Induced Senescence and Fibrosis in Fibroblasts (Shi L, Ding Z, et al., 2025).
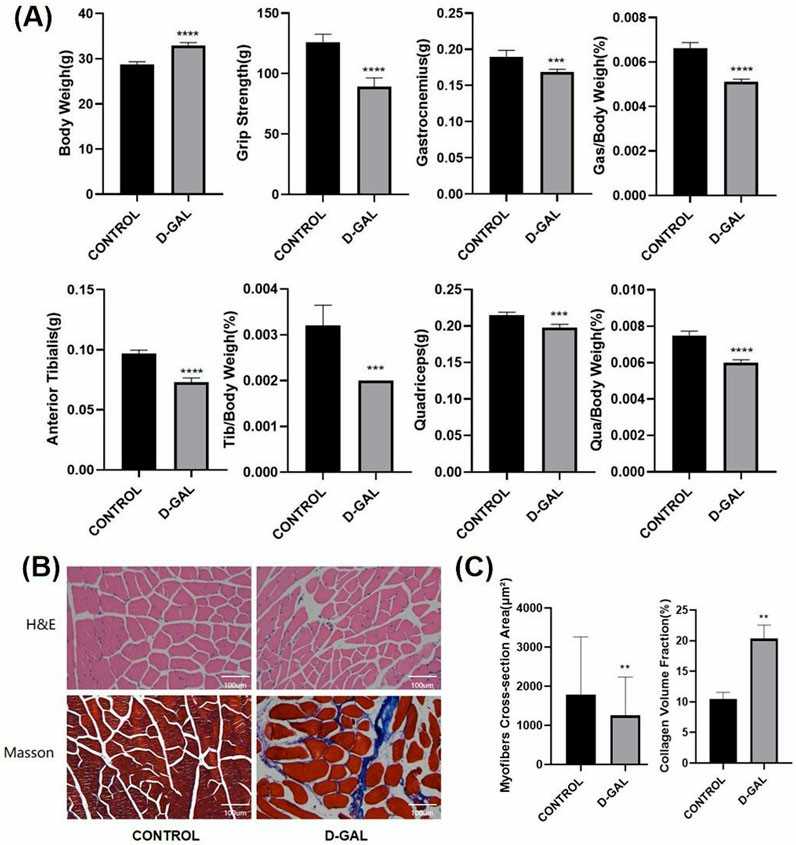 Fig. 2. D-Gal Triggers Muscle Atrophy and Fibrosis in Mice (Shi L, Ding Z, et al., 2025).
Fig. 2. D-Gal Triggers Muscle Atrophy and Fibrosis in Mice (Shi L, Ding Z, et al., 2025).
Em Inhibits TGF-Β1/Smad Signaling in Skeletal Muscle Fibroblasts
Age-related muscle loss and fibrosis in sarcopenia are difficult to treat. SGLT2 inhibitor empagliflozin (Em) has been shown to have anti-fibrotic effects in various organs. Here, Huang et al. explored the possibility that Em alleviates TGF-β1-induced fibroblasts and muscle fibrosis in aging mice through the AMPKα/MMP9/TGF-β1/Smad signaling pathway, as a potential treatment for sarcopenia.
To assess the effects of Em and TGF-β1 on skeletal muscle fibroblasts, cells were treated with TGF-β1 (1-40 ng/ml; Fig. 3a) or Em (0.5-20 μM; Fig. 3b). CCK8 assays confirmed no cytotoxicity at tested concentrations, leading to selection of 10 ng/ml TGF-β1 and 1 μM Em for subsequent experiments. Western blot results showed that TGF-β1 increased the expression of fibrosis markers in cells compared with the NC group, while the addition of Em reversed this effect (TGF-β1+Em group; Fig. 3c). Immunofluorescence analysis also demonstrated increased COL1A1, COL3A1 and α-SMA expression in TGF-β1-treated cells compared with the NC group, while Em attenuated this effect (Fig. 4a-c). TGF-β1/Smad pathway detection showed that Em inhibited TGF-β1-induced upregulation of p-Smad2/3 expression (Fig. 3d).
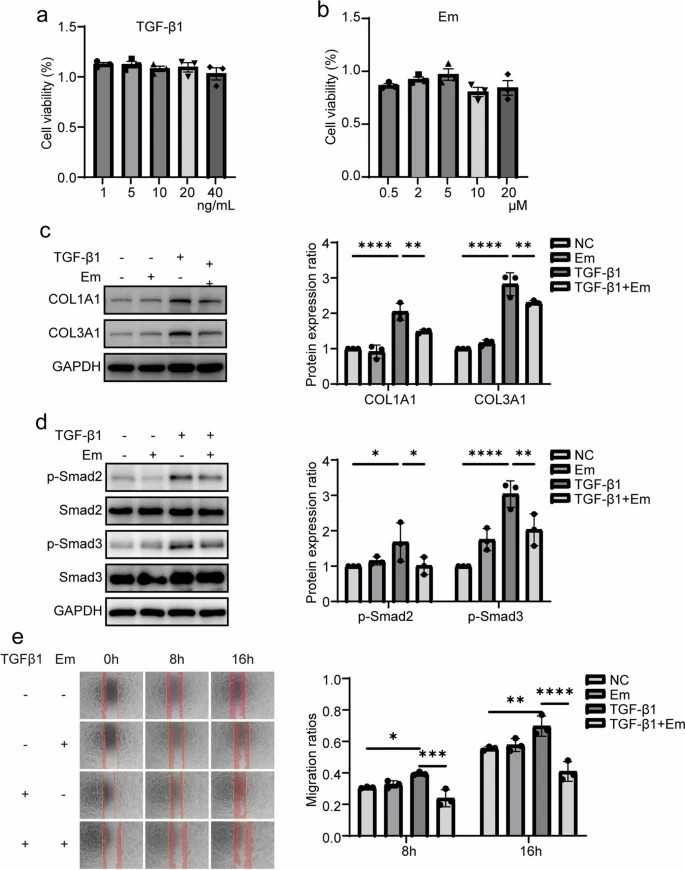 Fig. 3. Em can inhibit TGF-β1/Smad signaling in skeletal muscle fibroblasts, thereby suppressing fibrotic expression and reducing the migration ability of these fibroblasts (Huang Q, Chen J, et al., 2024).
Fig. 3. Em can inhibit TGF-β1/Smad signaling in skeletal muscle fibroblasts, thereby suppressing fibrotic expression and reducing the migration ability of these fibroblasts (Huang Q, Chen J, et al., 2024).
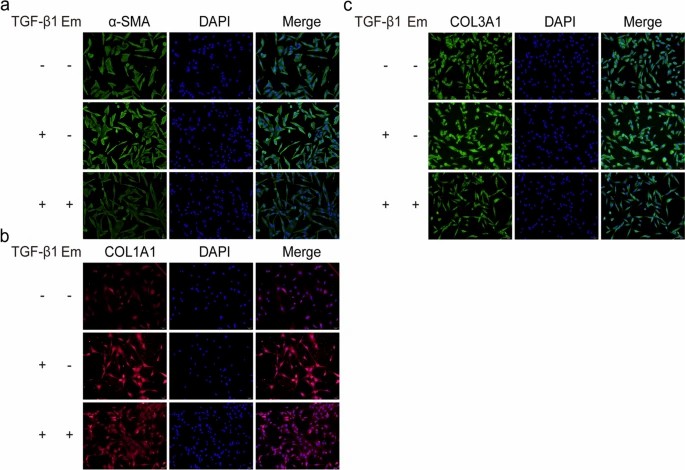 Fig. 4. Em inhibits fibrotic expression in skeletal muscle fibroblasts (Huang Q, Chen J, et al., 2024).
Fig. 4. Em inhibits fibrotic expression in skeletal muscle fibroblasts (Huang Q, Chen J, et al., 2024).
Ask a Question
Write your own review
- You May Also Need