Mouse Endometrial Epithelial Cells
Cat.No.: CSC-C5325S
Species: Mouse
Source: Endometrium; Uterus
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse endometrial epithelial cells from Creative Bioarray are isolated from the mouse uterine tissue. The method we use to isolate mouse endometrial epithelial cells was developed based on a combination of established and our proprietary methods. The mouse endometrial epithelial cells are characterized by immunofluorescence with antibodies specific to pan-cytokeratin (PCK). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse Endometrial Epithelial Cells (MEECs) are epithelial cells that originate in the endometrium of the mouse uterus. The endometrium is the inner lining of the uterus and contains two primary sections: the epithelium and the stroma. The epithelial cells are the outermost layer of the endometrium and line the uterine cavity while the stromal cells situate beneath the epithelium. Hormones such as estrogen and progesterone control the positioning of MEECs within the uterus. These cells undergo periodic changes during the menstrual cycle, including proliferation, secretion, and shedding, to accommodate the needs of pregnancy or menstruation. They also secrete proteins and hormones that have roles in signaling from the embryo to the mother or vice versa, like in the case of trophoblast cells. MEECs are commonly used in the research of endometrial diseases, including endometriosis and adenomyosis.
Transcriptome Analysis Reveals Hub Genes and Ihh and KLF9 Axis in the Endometrial Restoration in IUA Mice
Intrauterine adhesion (IUA) is a condition characterized by endometrial injury and fibrosis, leading to menstrual disturbances and infertility. Current treatments like hysteroscopic adhesiolysis have high recurrence rates and lack solid efficacy data. In the recent study, Zhou's team isolated and expanded conditional reprogrammed (CR) mouse endometrial epithelial cells (CRCs). Treating IUA mice with CRCs restored the endometrial morphology and structure and significantly improved the pregnancy rate.
To study the underlying molecular mechanism of CRCs in restoring the endometrium, uterine horns in the transplanted, injured, and sham-operated mice were collected 7 days after surgery for RNA-seq. The results of hierarchical clustering and PCA analysis showed that the samples in the transplanted group were closer to the control group rather than the injured group (Fig. 1A). It indicated that the samples of transplanted mice had similar gene expression patterns to the sham-operated group, which were obviously different from the injured group (Fig. 1B). The results of GO analysis for the DEGs in the injured group showed the changed pathways were mainly enriched in gland development, steroid metabolic process, epithelial cell proliferation, connective tissue development, and cellular hormone receptor activity (Fig. 1C). The analysis of gene regulatory network identified the hub genes of Ihh and KLF9 in endometrial restoration by CRCs in IUA mice (Fig. 1D). qRT-PCR verified that Ihh and KLF9 were upregulated in the injured group (Fig. 1E). Ihh is a member of the Hedgehog family, which plays important roles in the development of many tissues. Taken together, these findings suggest that Ihh and KLF9 are key genes for endometrial restoration in IUA mice.
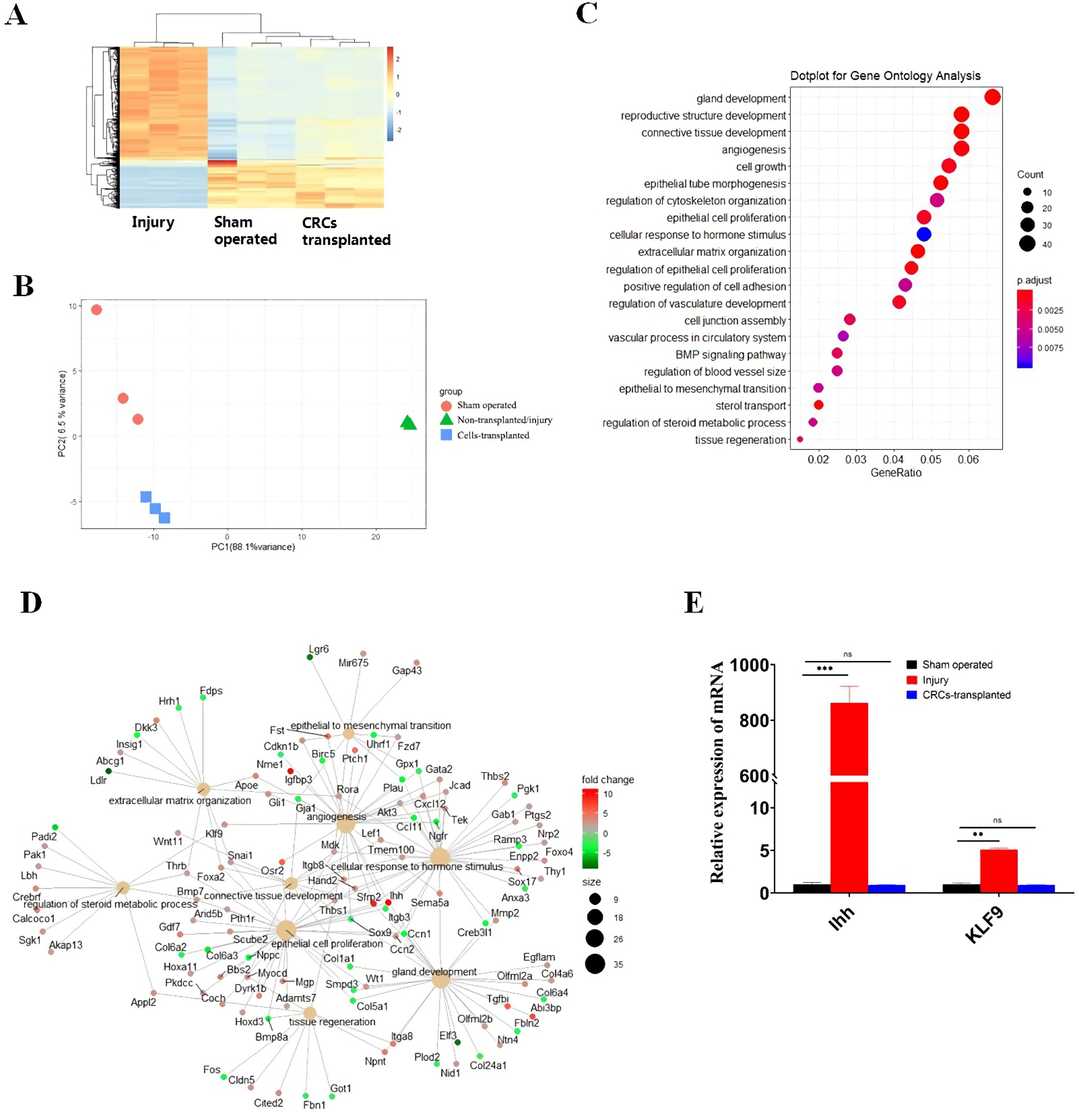 Fig. 1. Transcriptomeanalysis reveals hub genes and Ihh and KLF9 axis in the repair of uterine endometrium by CRCs transplantation (Zhou X, Kang Y, et al., 2022).
Fig. 1. Transcriptomeanalysis reveals hub genes and Ihh and KLF9 axis in the repair of uterine endometrium by CRCs transplantation (Zhou X, Kang Y, et al., 2022).
MEECs Maintain Normal Biological Characteristics in 3D Matrigel Cultures
Intrauterine adhesions (IUAs) cause significant menstrual and fertility issues, with existing treatments having high recurrence rates. Xia's team investigated the use of conditionally reprogrammed (CR) endometrial epithelial cells to address IUA. Mouse endometrial epithelial cells (MEECs) were isolated and cultured using the CR method. Their biological characteristics and tissue-specific differentiation potential were assessed.
Normal cells can arrest growth when exposed to mutagens, while tumor cells lose this function. They analyzed the response of MEECs to DNA damage-induced growth arrest. MEECs were treated with actinomycin D (Act D) for 24 h. Figure 2A shows that p53 and its downstream effector p21 were upregulated in MEECs treated with Act D, unlike in HeLa cells. They then evaluated the transforming property of MEECs using soft agar. MEECs did not form colonies in soft agar (Fig. 2B), while HeLa cells did. They also assessed the differentiation potential of MEECs using matrigel 3D culture. Figure 2C shows that MEECs formed well-organized spheres, while HeLa cells formed irregular aggregates. These results demonstrate that MEECs maintain normal biological characteristics and differentiation potential, which is crucial for treating IUA.
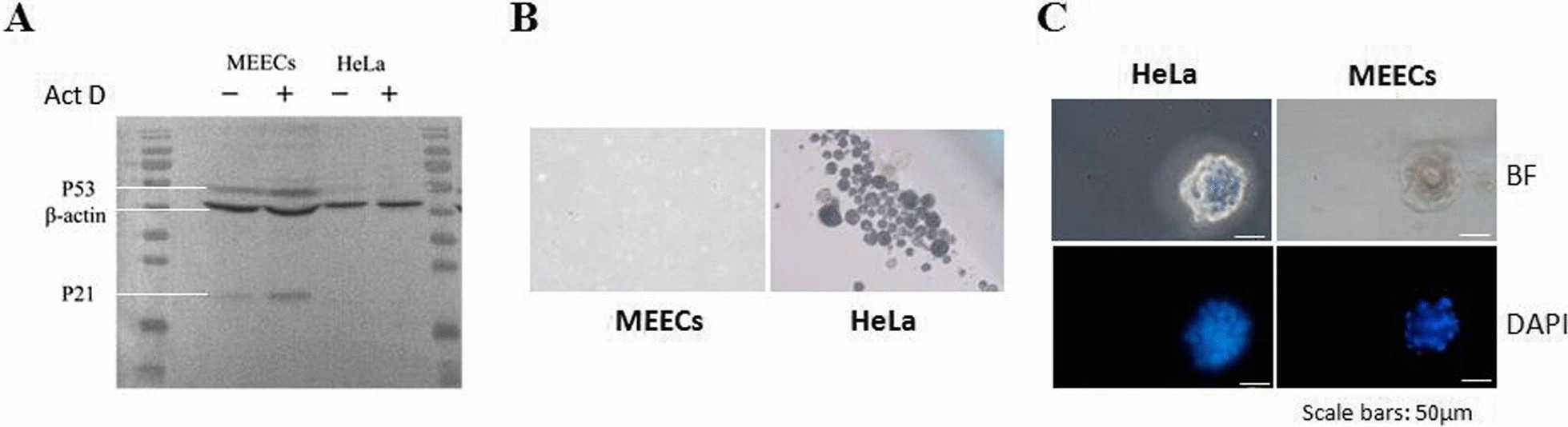 Fig. 2. Characterization of normal biological features of MEECs (Xia S Y, Wu M, et al., 2022).
Fig. 2. Characterization of normal biological features of MEECs (Xia S Y, Wu M, et al., 2022).
Ask a Question
Write your own review
- You May Also Need