- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The hFOB 1.19 cell line originates from human fetal osteoblasts and maintains primary osteoblast-like characteristics. These cells can transform into mature osteoblasts and show strong cell division potential. The cells produce osteogenic markers including alkaline phosphatase (ALP) and osteocalcin (OCN) which enable them to develop mineralized nodules when exposed to specific inducive conditions. Research shows that these cells contribute to mineralization by releasing matrix metalloproteinases (MMPs) and proteins related to calcification such as TNAP and Anx2. hFOB 1.19 cells show multipotent differentiation capabilities because they can transform into different mesenchymal progenitor cell types such as chondrocytes and adipocytes.
Researchers utilize hFOB 1.19 cells to determine how different materials including titanium alloys and bioceramics interact with bone formation and support bone regeneration. Researchers utilize this cell line to examine how different drugs affect osteogenic differentiation and mineralization processes through compounds like baicalin, melatonin, and paclitaxel. Research on disease mechanisms and therapeutic approaches for conditions like osteoporosis and osteosarcoma involves the use of hFOB 1.19 cells.
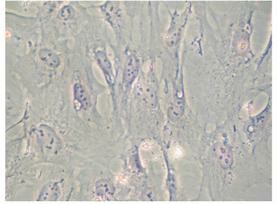 Fig. 1. Morphology of hFOB 1.19 cells (Mroczek J, Pikula S, et al., 2022).
Fig. 1. Morphology of hFOB 1.19 cells (Mroczek J, Pikula S, et al., 2022).
Characterization of the Mineralization Process in Human Fetal Osteoblastic Cell Line (hFOB 1.19 Cells) and Osteosarcoma Cell Line (Saos-2 Cells)
Osteoblast and chondrocyte-mediated mineralization employs matrix vesicles (MVs) rich in TNAP and annexins, essential for apatite formation. Bozycki et al. compared mineralization capabilities of hFOB 1.19 osteoblastic cells and Saos-2 osteosarcoma cells.
Human fetal osteoblastic hFOB 1.19 and osteosarcoma Saos-2 cells were cultured for seven days in media without (Resting, R) or with 50 μg/mL ascorbic acid (AA) and 7.5 mM β-glycerophosphate (β-G) (Stimulated, S). Resting cells were elongated (Fig. 1A and B, arrows). AA and β-GP treatment caused hFOB 1.19 cells to halve their longest axis (Fig. 1C and 1E), while Saos-2 cells' length remained unchanged (Fig. 1D and E). Alizarin red-S/cetyl pyridinium chloride (AR-S/CPC) analysis showed stimulated cells formed more calcium nodules (hFOB 1.19—twice, Saos-2—nearly thrice) versus resting cells (Fig. 2A and B). Levamisole (Lev), a TNAP inhibitor, largely blocked nodule formation (Fig. 2A and B). K201 increased amorphous calcium complexes in resting cells (Fig. 2A and B). TNAP activity was lower in hFOB 1.19 than Saos-2 cells (Fig. 2C and D), increasing in stimulated conditions (Fig. 2C and D). Lev reduced TNAP activity to basal levels, while K201 had minimal impact (Fig. 2C and D).
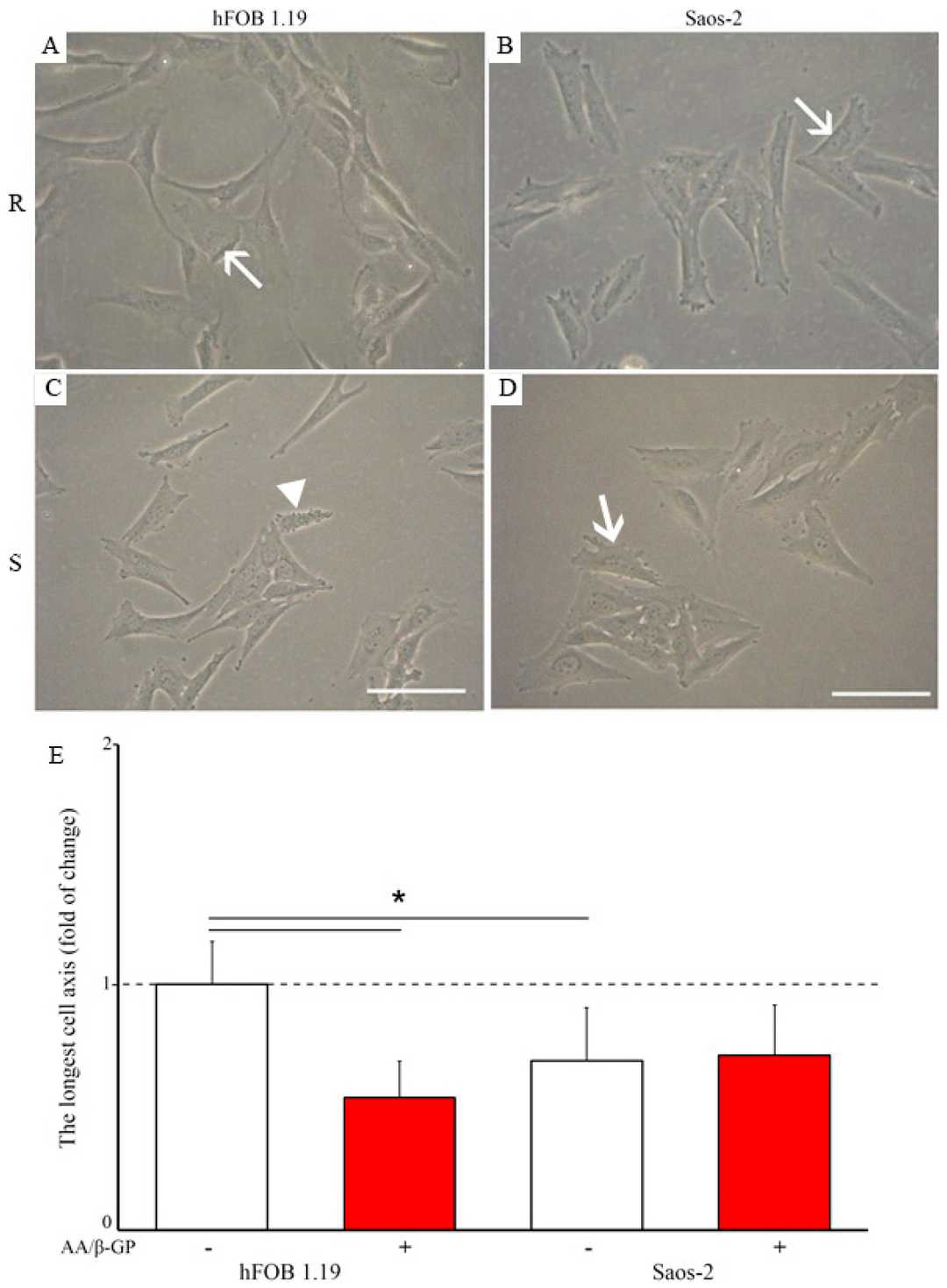 Fig. 1. Morphology of hFOB 1.19 (A,C) and Saos-2 (B,D) cells in resting (A,B) and stimulated (C,D) conditions (Bozycki L, Mroczek J, et al., 2021).
Fig. 1. Morphology of hFOB 1.19 (A,C) and Saos-2 (B,D) cells in resting (A,B) and stimulated (C,D) conditions (Bozycki L, Mroczek J, et al., 2021).
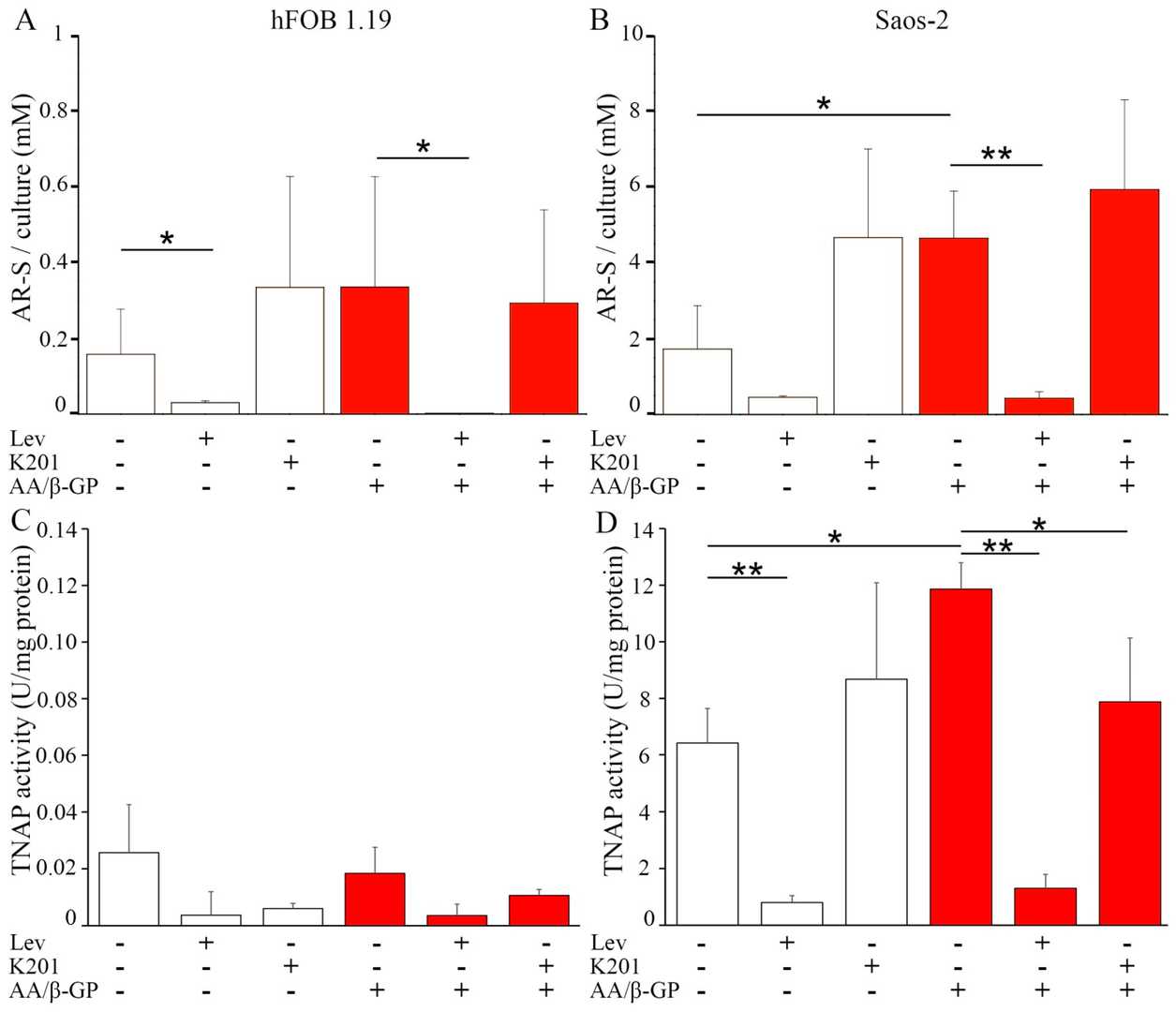 Fig. 2. Mineralization level of hFOB 1.19 (A,C) and Saos-2 (B,D) cells in resting conditions (white) or after seven-day stimulation with AA and β-GP (red) (Bozycki L, Mroczek J, et al., 2021).
Fig. 2. Mineralization level of hFOB 1.19 (A,C) and Saos-2 (B,D) cells in resting conditions (white) or after seven-day stimulation with AA and β-GP (red) (Bozycki L, Mroczek J, et al., 2021).
Determination of the Effects of SeMet on Osteogenic Differentiation Using Alizarin Red Dye and Image Analysis
Osteoporosis stands as a widespread condition that causes fractures and bone metabolism factors affect its development. Multiple scientific studies demonstrate that selenium serves as an essential trace element with beneficial effects on bone health. Sahin et al. investigated if selenomethionine (SeMet), a selenium variant, accelerates the differentiation process of osteoblastic hFOB cells.
Two experimental groups were created using SeMet concentrations of 5, 10, 15, and 25 μM with one group including osteogenic differentiation cocktail (OD +) and the other excluding it (OD ?). At the end of the 28th day, both models were stained with alizarin red, and calcium deposits were examined. It was observed that calcium deposits increased in a dose-dependent manner in both the OD(?+) and OD(??) groups in which SeMet was applied (Fig. 3).When alizarin red stainings were analyzed with the ImageJ program, it was determined that SeMet increased the amount of calcium deposits in a concentration-dependent manner in both experimental setups (SeMet concentrations?+?OD(?+) or OD(??)), and in 5–10-15 and 25 μM SeMet?+?OD(?+) administrations, each concentration was significant compared to the control (p?
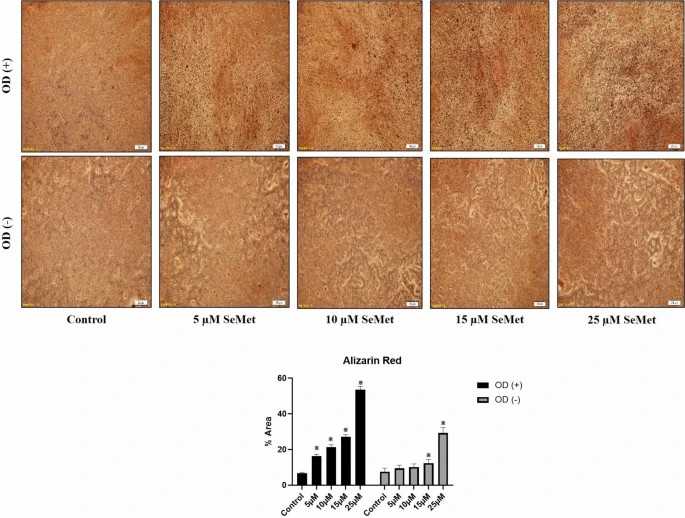 Fig. 3. Calcium deposit results (alizarin red staining) (Sahin E, Arafat M, et al., 2024).
Fig. 3. Calcium deposit results (alizarin red staining) (Sahin E, Arafat M, et al., 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells