Cynomolgus Monkey Dermal Fibroblasts
Cat.No.: CSC-C4723L
Species: Monkey
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Cynomolgus monkey dermal fibroblasts are isolated from the dermal tissue of cynomolgus monkey. Fibroblasts are the primary cell type in the dermis and are involved in a range of physiological and pathological processes. For instance, fibroblasts are the main producers of the extracellular matrix (ECM) of the skin and can secrete various types of collagens (such as type I and III), elastin, fibronectin, and other ECM components to provide structural support for the skin. When tissues become inflamed or injured, fibroblast cells increase their activity by releasing numerous cytokines and chemokines which aid in tissue repair. Fibroblasts also possess immune regulatory functions, able to secrete various cytokines (such as IL-6, TGF-β, IL-10, etc.), regulate the activity of T cells, B cells, and macrophages, and influence immune responses. In addition, fibroblasts can also serve as antigen-presenting cells (APCs) and participate in immune responses in lymphoid tissues. In recent years, studies have shown that fibroblasts also exhibit some characteristics of stem cells, with the ability to self-renew and differentiate into multiple cell types under certain conditions. For example, fibroblasts can obtain characteristics similar to mesenchymal stem cells (MSCs) through epigenetic regulation and exhibit multipotent differentiation potential. Fibroblasts can also be reprogrammed into induced pluripotent stem cells (iPSCs), providing new tools for stem cell research and regenerative medicine.
Generation and Characterization of cynHLCs from Cynomolgus Monkey Dermal Fibroblasts
This study presents the development of a Pc in vitro infection model using stem cell-derived hepatocytes from Macaca fascicularis. Cynomolgus iPS (cynIPS) cells were generated from two Cynomolgus Monkey dermal fibroblasts by reprogramming, using Sendai viruses bearing the transcription factors Oct3/4, Sox2, Klf4, and c-Myc. Pluripotent colonies appeared two to three weeks after infection and were kept on irradiated mouse embryonic fibroblasts. After several passages, the iPS cells were transferred on Matrigel, but spontaneous differentiation occurred on this feeder-less system (Fig. 1). Further investigations showed that a higher concentration of bFGF (basic fibroblast growth factor) was essential to maintain pluripotency (Fig. 1). Following the clonal expansion phase, clones exhibiting typical human embryonic stem cell-like morphology (flat and tightly packed colonies of round cells with large nuclei) were selected and characterized by immunofluorescence (Fig. 1). Based on this result the best clone (#13) was selected and checked for karyotype (Fig. 1), and differentiation potential into the three germ layers (Fig. 1).
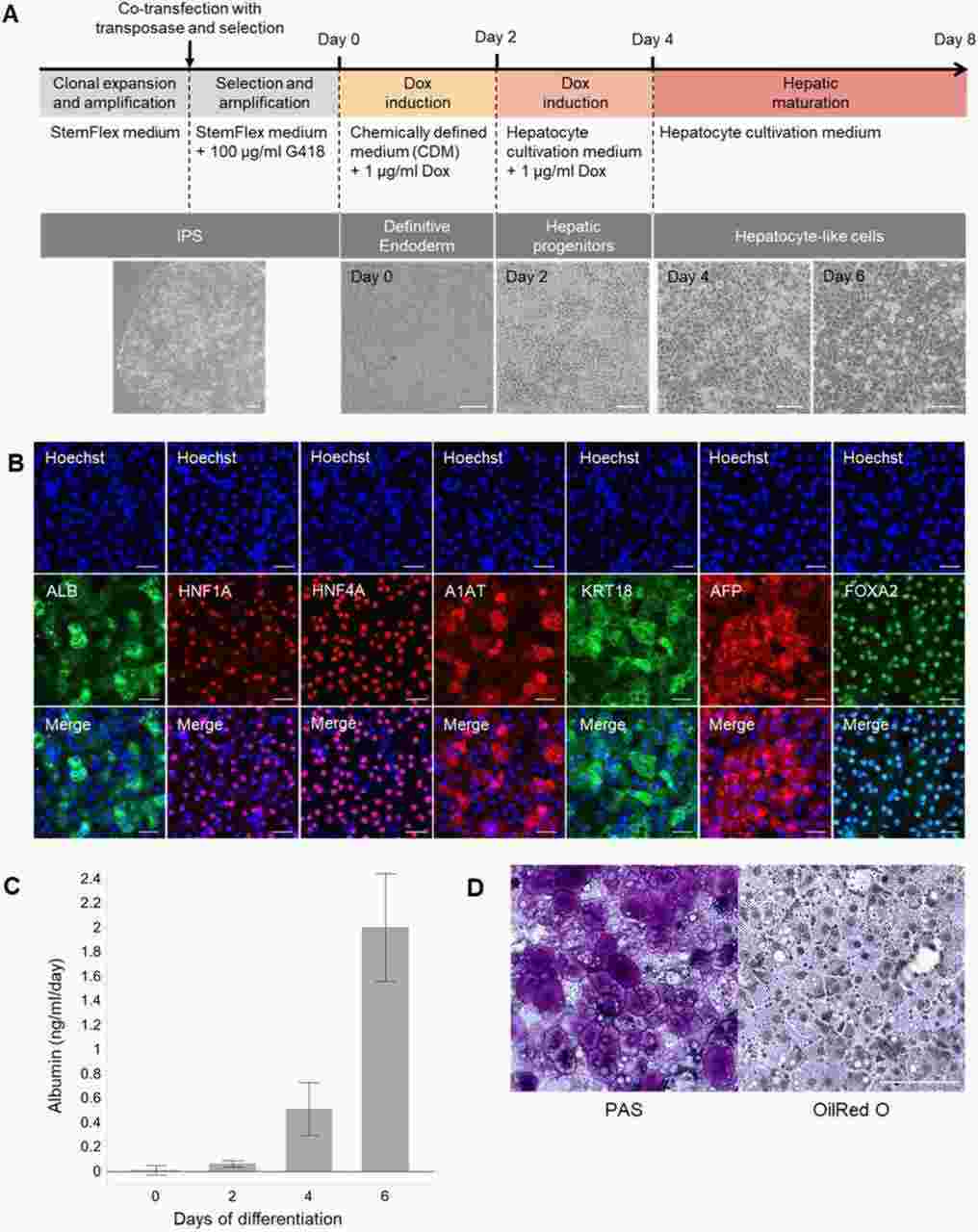 Fig. 1. Generation and characterization of cynHLCs (Pellisson M, Zeeman A M, et al., 2021).
Fig. 1. Generation and characterization of cynHLCs (Pellisson M, Zeeman A M, et al., 2021).
CERV1 Infectivity in Human and Primate Cell Lines
Endogenous retroviruses (ERVs) are ancient viral sequences in the mammalian genome, providing insights into virus–host co-evolution. Chimpanzee endogenous retrovirus 1 (CERV1), an extinct gamma retrovirus, has circulated in primates for about 10 million years. AbuEed et al. aim to understand how CERV1 was transmitted and endogenized in primates.
In the present study, CERV1 was found to be a viral interference group of PERV-A and PERV-A/C. As CERV1, PERV-A, and PERV-A/C utilize the same receptor, human and primate cell lines were assessed for susceptibility by Env-pseudotyped viruses (Fig. 2). CERV1 was found to infect humans (293T, HeLa, HT1080, and Hs68 cells), chimpanzees (Chimp-0363M cells), and African green monkeys (Cos7 and Vero cells), although not NCMDF (normal cynomolgus monkey dermal fibroblasts). Contrastingly, PERV-A was found to infect HEK293T and HeLa cells but not other lines. Moreover, with the exception of Vero cells, PERV-A/C was established to show infectivity in the human and primate cell lines assessed in this study. These findings indicate that despite using the same viral receptor, there are differences in susceptibility with respect to viral cell entry.
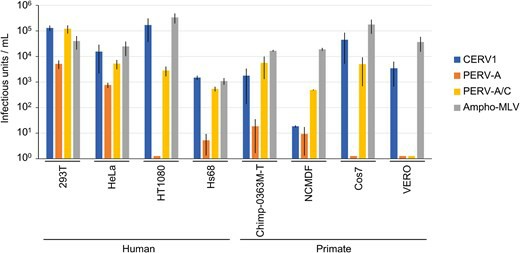 Fig. 2. Comparison of the infectivity of CERV1, PERV-A, and PERV-A/C in human and primate cell lines (AbuEed L, Miyake A, et al., 2025).
Fig. 2. Comparison of the infectivity of CERV1, PERV-A, and PERV-A/C in human and primate cell lines (AbuEed L, Miyake A, et al., 2025).
Ask a Question
Write your own review