Murine endothelial cells
Cat.No.: CSC-C1746
Species: Mouse
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
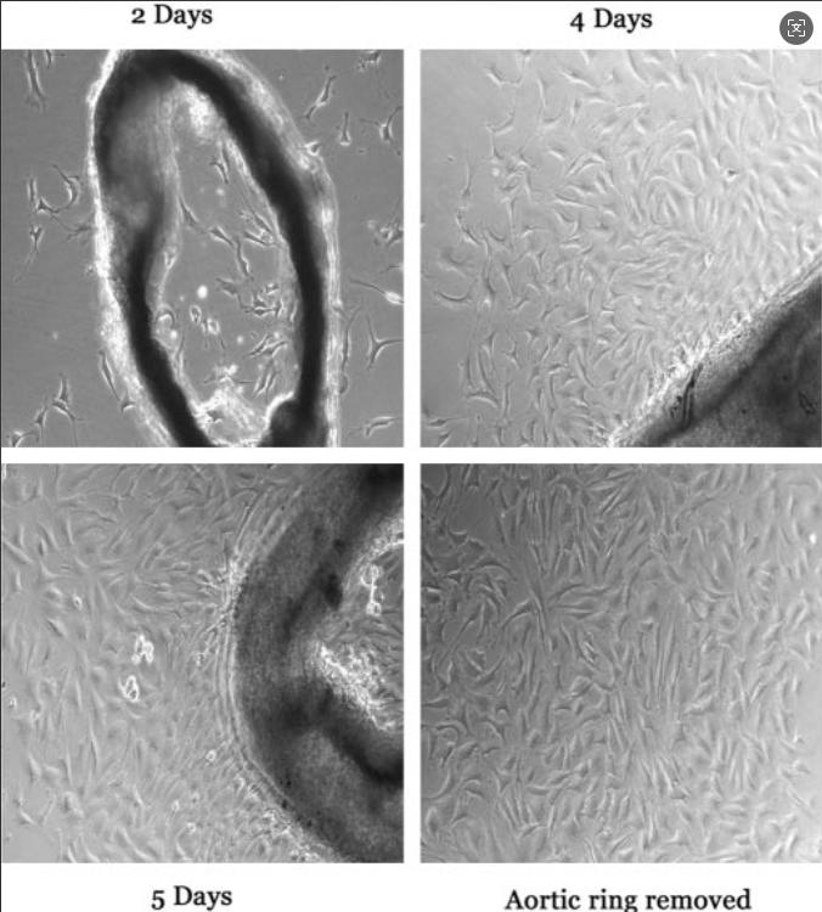
Murine endothelial cells are primary or immortalized primary cells that are isolated from the vascular lining of mice (Mus musculus). They are used to understand vascular biology and pathology. They are often isolated from tissues like aorta (i.e. MAECs), brain microvasculature (i.e. bEnd.3), lung, or liver and have morphological and functional characteristics depending on the tissue of origin. For example, aortic endothelial cells are long and tubular while microvascular endothelial cells tend to form cobblestone-like monolayers.
Murine endothelial cells help in controlling vascular permeability, angiogenesis, immune cell activity, and coagulation through markers such as CD31, VE-cadherin, and eNOS. They require specific media like EGM-2 supplemented with growth factors and need to be coated with extracellular matrix proteins (e.g. gelatin) to adhere to surfaces. They are commonly used for atherosclerosis, tumor angiogenesis, blood-brain barrier disruption, or inflammatory diseases. Immortalized cell lines (i.e. bEnd.3, SVEC4-10) allow for extended research, while primary cells offer a more physiologically relevant model.
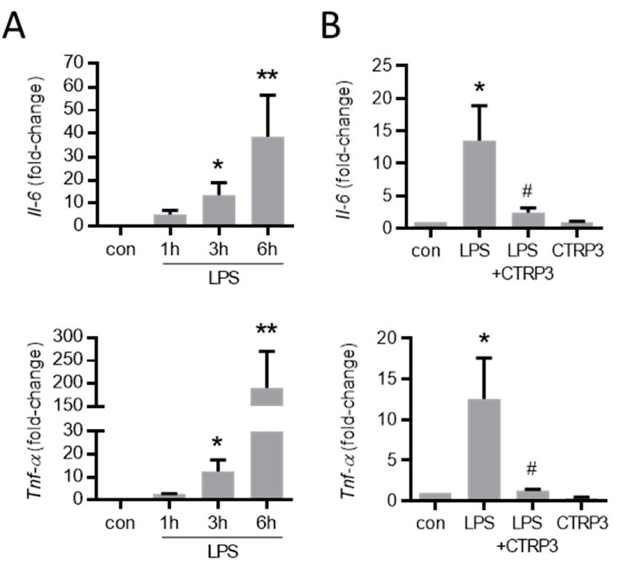
LPS-Induced Cytokine Expression in Endothelial Cells Is Inhibited by CTRP3
CTRP3 is an adipokine associated with obesity and type 2 diabetes that has been found to have anti-inflammatory effects in adipocytes and monocytes/macrophages. Its role in endothelial cells during endotoxemia, however, is still unknown. To this end, Schmid et al. tested whether CTRP3 has anti-inflammatory effects in endothelial cells treated with LPS regarding cytokine production, expression of adhesion molecules, and monocyte adhesion.
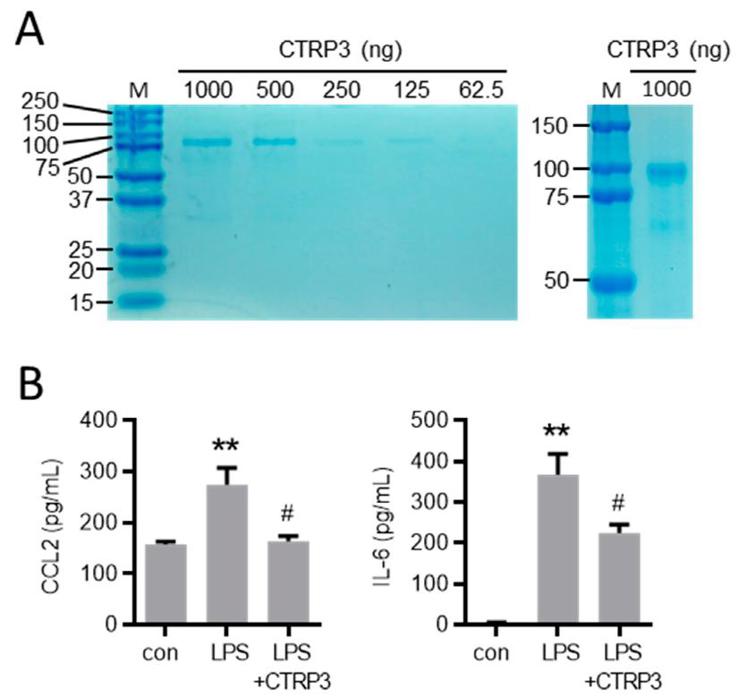
In line with their previous findings, LPS (50 ng/mL) induced a strong expression of inflammatory cytokines like interleukin-6 (Il-6) and the tumor necrosis factor-α (TNF-α) in murine endothelial (MyEND) cells after 3 h (Fig. 1A). To study the effects of exogenous CTRP3 in this setting, the authors used recombinant CTRP3 that was expressed using the baculovirus expression system in insect cells as described previously. Recombinant CTRP3 migrated as a trimeric molecule at < 100 kDa on SDS-polyacrylamide gels (Fig. 2A) and blocked LPS-induced CCL2 and IL-6 release from THP-1 cells in activity assays (Fig. 2B). Pre-incubation of MyEnd cells with CTRP3 (30 min) led to a significant reduction in the LPS-induced expression of Il-6 and Tnf-α after 3 h, while CTRP3 alone had no effect on the expression of cytokines (Fig. 1B).
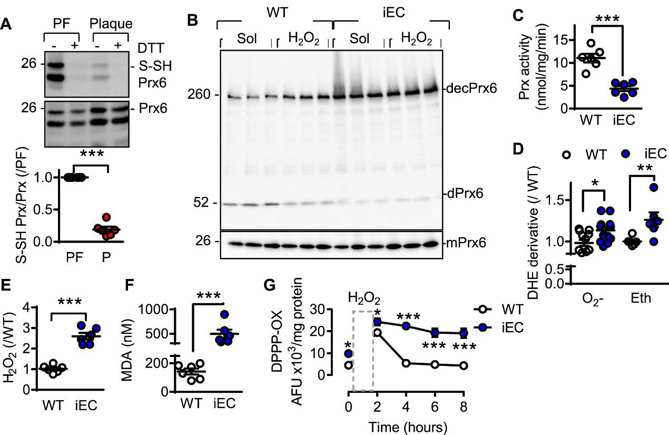
Cystathionine γ Lyase and the Sulfhydration of Peroxiredoxin 6 in Endothelial Cells
Cystathionine γ lyase (CSE) is the key enzyme for H2S production in endothelial cells and confers atheroprotection. Whether and how shear stress influences CSE expression, and how this would in turn modulate endothelial redox homeostasis was, however, unknown. Bibli et al. examined fluid shear stress regulation of CSE expression through KLF2 and miR-27b and its antioxidant properties against oxidative damage and lipid peroxidation in endothelial cells.
Endogenous H2Sn have emerged as important regulators of endothelial redox homeostasis. Given that H2Sn elicit their biological effects through protein sulfhydration, they first interrogated sulfhydrated proteins in murine endothelial cells derived from plaque-free and plaque-containing arteries. Only eight redox-related proteins (GO: 0045454) were identified. Peroxiredoxin 6 (Prx6) was the most abundant and in cells from plaque-free (H2S-high) endothelium, its reactive Cys47 was sulfhydrated. This finding was validated by biotin switch assays in human plaque-free vessels (Fig. 3A) as well as wild-type murine endothelial cells. Prx6 sulfhydration was not detected in the endothelial cells of CSEiEC mice. CSEiEC cells showed a loss of sulfhydration and exhibited increased Prx6 decamer formation (Fig. 3B), a hyperoxidized form of Prx6. Supplementation of H2O2 (100 μmol/L) further augmented decameric Prx6 in wild-type cells but failed to do so in CSE-deficient cells, indicating that the latter were already oxidized at baseline. Prx6 activity was also profoundly lower in CSEiEC cells compared with wild-type (Fig. 3C).
Ask a Question
Write your own review