Mouse Red Blood Cells
Cat.No.: CSC-C3101
Species: Mouse
Source: Blood
Cell Type: Red Blood Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse red blood cells (RBCs), also known as erythrocytes, are terminally differentiated, enucleated cells that circulate in the blood vessels and function in oxygen transport. Mouse RBCs develop from hematopoietic stem cells in the bone marrow via erythropoiesis, and mature erythrocytes can be identified by their distinct biconcave disc shape (7-8 μm in diameter) and expression of surface markers such as Ter-119. The specialized structure of red blood cells includes cytoplasm that contains 95% hemoglobin by dry weight while the cytoskeleton consists of spectrin along with other proteins. These features allow RBCs to efficiently transport oxygen and carbon dioxide, and to deform and squeeze through the narrow capillaries in the microvasculature.
Mouse RBCs survive between 40 and 60 days before removal from blood circulation by the spleen and liver. Several hematology research applications utilize mouse RBCs for modeling diseases such as phenylhydrazine-induced anemia, Plasmodium infections in malaria research, and hemoglobinopathies through transgenic mouse models for sickle cell disease. They are also used in studies of biomechanics (e.g., RBC membrane elasticity), and toxicology (e.g., screening for hemolytic compounds).
Mouse RBCs fulfill basic functions similar to human red blood cells but possess crucial distinctions that researchers need to consider during translational studies. These include differences in size, lifespan, and hemoglobin composition. Recent and potential applications of mouse RBCs include RBC-derived extracellular vesicles and RBC membrane-coated biomimetic drug carriers.
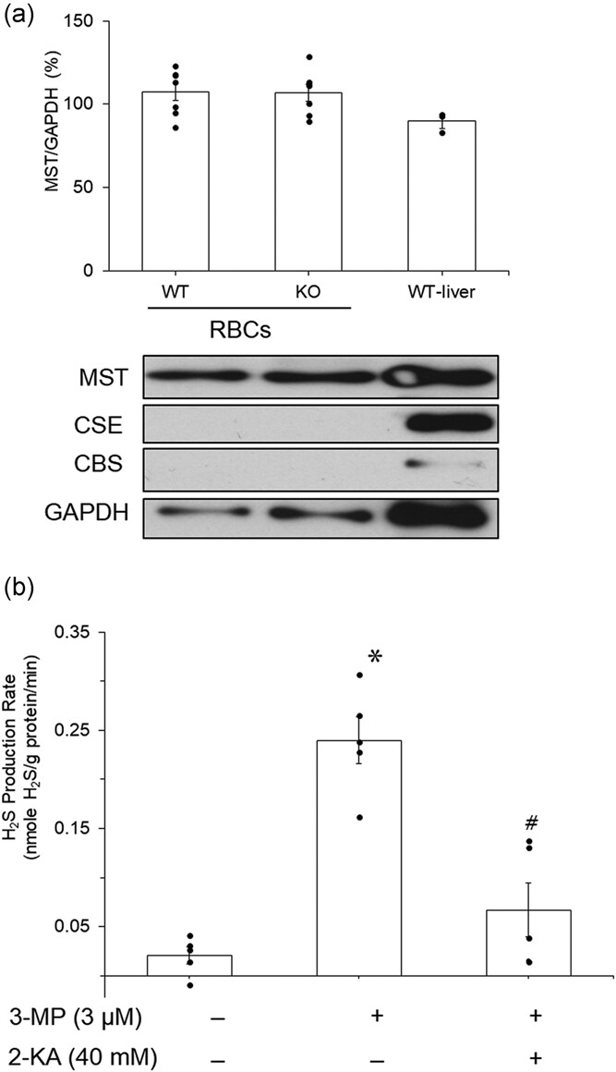
Mouse RBCs Synthesize H2S via MST and H2S Stimulates Glycolysis
Hydrogen sulfide (H2S) is a gasotransmitter involved in physiological processes. While its role in promoting mitochondrial energy production under hypoxia is known, its effect on anaerobic glycolysis is not. Mammalian red blood cells (RBCs), which lack mitochondria, rely on glycolysis for energy. Wondimu et al. used Western blot analysis to identify H2S-generating enzymes in mouse RBCs and investigated the effect of H2S on glycolysis-mediated ATP production.
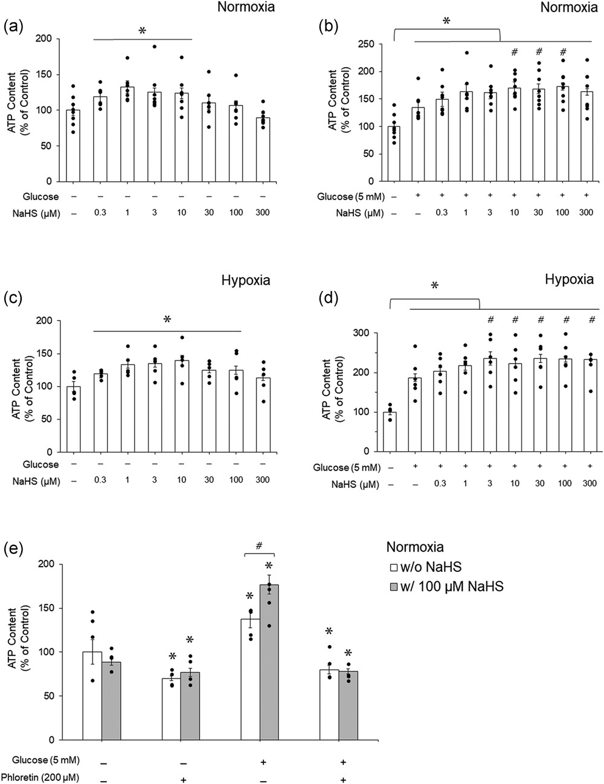
Western blot analysis showed the presence of MST protein but not CSE and CBS proteins in mouse RBCs (Fig. 1a). 3-MP enhanced H2S production in mouse RBC lysates (Fig. 1b), which was suppressed by 2-KA, an MST inhibitor. 0.3-10 µM NaHS significantly increased ATP levels in mouse RBCs with no extracellular glucose under normoxia (Fig. 2a). Similarly, 0.3-100 μM NaHS significantly increased ATP levels in mouse RBCs with no extracellular glucose under hypoxia (Fig. 2c). The maximal effect was seen with 1 µM under normoxia and 10 µM under hypoxia. There was no further increase with higher concentrations of NaHS. Extracellular glucose (5 mM) significantly increased ATP levels (Fig. 2b). NaHS further enhanced ATP levels under both normoxia and hypoxia in the presence of extracellular glucose (Fig. 2b, d). The effect of NaHS on ATP levels was blocked by phloretin, an inhibitor of glucose transporter (Fig. 2e).
Ask a Question
Write your own review