Mouse Bone Marrow Mononuclear Cells
Cat.No.: CSC-C4463X
Species: Mouse
Source: Bone Marrow
Cell Type: Mononuclear Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Bone Marrow Mononuclear Cells (BM-MNCs) represent a heterogeneous population isolated from murine bone marrow through density gradient centrifugation, comprising hematopoietic stem/progenitor cells (HSPCs), mesenchymal stromal cells, lymphocytes, monocytes, and endothelial progenitors. Morphologically BM-MNCs can be small, densely stained lymphocytes (6-8 μm), or larger monocytes (12-20 μm) with kidney-shaped nuclei. BM-MNCs are capable of multilineage differentiation (myeloid, erythroid, osteogenic), can secrete cytokines and display immunomodulatory effects (IL-10, TGF-β) and paracrine secretion (VEGF, HGF) to mediate tissue repair and regeneration. BM-MNCs are commonly used for studying hematopoiesis, and are a model for leukemogenesis, aplastic anemia, and regenerative therapies. BM-MNCs are used in immunotherapy (CAR-T generation), toxicity testing and for stem cell biology. Mouse BM-MNCs have higher proliferation capacity and are more easily genetically modified than human BM-MNCs, and have greater physiological relevance than immortalized cell lines.
H2 Inhibits the Osteoclastogenesis of Mouse BMMCs
Hydrogen (H2) is one of the major biodegradation products of magnesium (Mg) alloys implanted for bony fracture healing and reconstruction; H2 thus plays a significant role in the regulation of local microenvironment and the biology of resident cells. Liu's team investigated how H2, with different concentrations and durations, regulates the osteoclastogenesis of mouse bone marrow mononuclear cells (BMMCs).
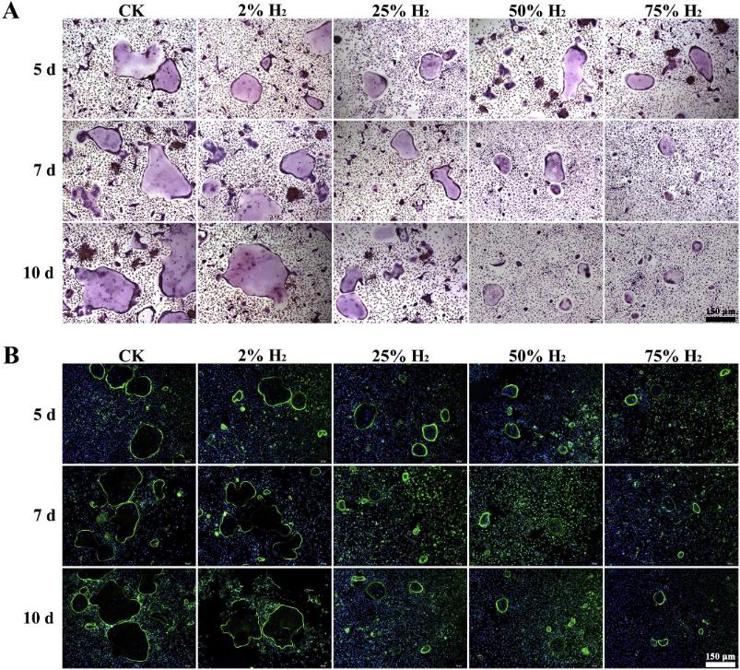
First, they measured the osteoclastogenesis of mouse BMMCs under five H2 concentrations (0%, 2%, 25%, 50%, and 75%) and three durations (5, 7, and 10 days) using TRAP staining, F-actin ring formation assay, pit formation assay, and RT-qPCR analysis. The number of multinucleated giant cells was significantly higher in the control and 2% H2 groups than in the 25%, 50%, and 75% H2 groups. Compared to the control group, 2% H2 did not significantly inhibit osteoclastogenesis after 5, 7, or 10 days of induction, while 25%, 50%, and 75% H2 did (Fig. 1A). Trap+ cells appeared after one day of induction, and Trap+ multinucleated cells appeared after three days. Their number peaked after seven days. The F-actin ring formation assay showed no differences in the number and size of F-actin rings between the 2% H2 and control groups after 5, 7, or 10 days of induction. However, these parameters were significantly reduced in the 25%, 50%, and 75% H2 groups, indicating significant inhibition of F-actin ring formation compared to the control group (Fig. 1B).
NaHS Mediated H2S Release, Enhances CXCR4 Expression and Chemotaxis to SDF-1Α
For successful transplantation of Hematopoietic Stem cells (HSCs), it is quite necessary that efficient homing, engraftment and retention of HSC self-renewal capacity takes place, which is often restricted due to inadequate number of adult HSCs. Here, Khanna et al. reported that short-term ex-vivo treatment of mouse bone marrow mononuclear cells (BMMNCs) to Sodium Hydrogen Sulfide (NaHS, hydrogen sulfide-H2S donor) can be used as a possible strategy to overcome such hurdle.
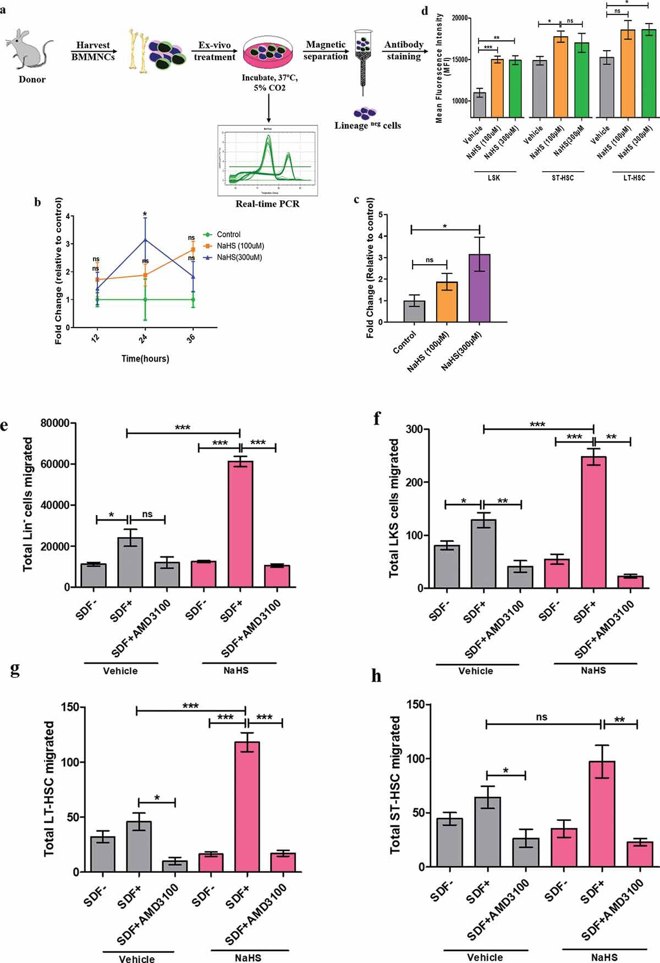
SDF-1α and CXCR4 guide HSPC migration. Elevated CXCR4 expression is vital for HSPC homing and engraftment. Here, CXCR4 mRNA and surface expression were measured in NaHS- and vehicle-treated cells (Fig. 2a). qRT-PCR showed maximal CXCR4 expression at 300 µM NaHS (3.4-fold). At this concentration, CXCR4 expression peaked at 24 hours (Fig. 2b, c). NaHS also increased CXCR4 surface expression, shown by higher MFI in LSK, ST-HSCs, and LT-HSCs (Fig. 2d). BAPTA-AM reduced CXCR4 expression, highlighting calcium's role. In a trans-well assay, linneg and LSK cells migrated toward SDF-1α, with NaHS-treated cells showing significantly more migration (Fig. 2e, f). Similar results were seen in ST-HSCs and LT-HSCs (Fig. 2g, h). AMD3100 blocked NaHS-induced migration, confirming CXCR4 mediation (Fig. 2g, h).
Ask a Question
Write your own review