
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HUC are isolated from the human bladder. HUC are cryopreserved at passage one and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HUC are characterized by immunofluorescent method with antibodies to cytokeratin-18, -19 and vimentin. HUC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HUC are guaranteed to further expand for 15 population doublings in the condition provided by Creative Bioarray.
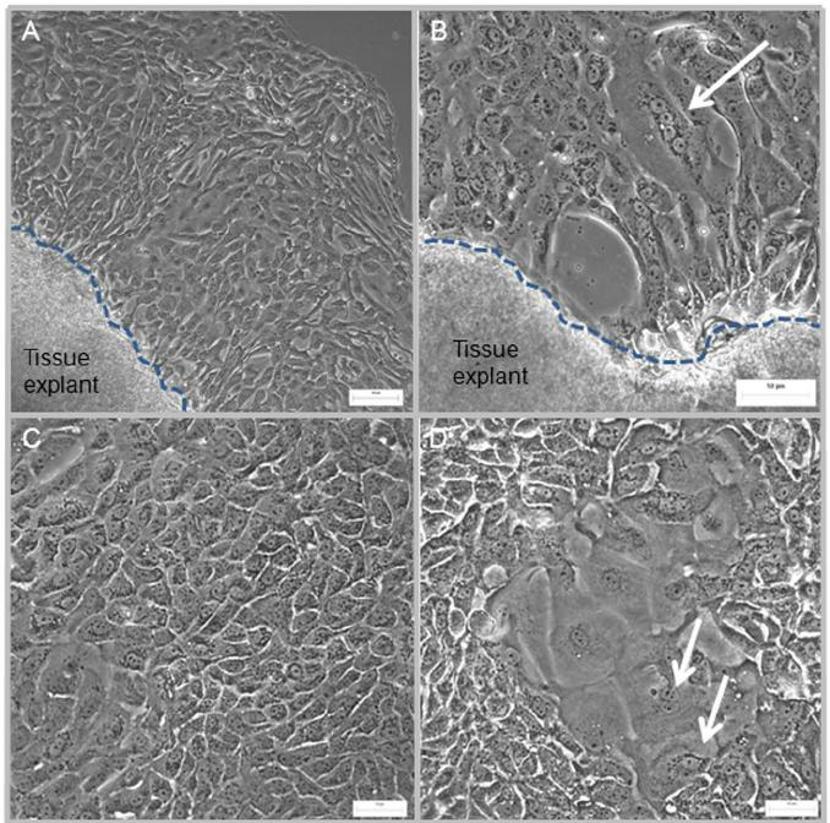
Human urothelial cells (HUCs) are epithelial cells found in the urinary tract, including the bladder, ureters, renal pelvis, and proximal urethra. They make up the urothelium, a stratified epithelium that serves as a barrier and is highly stretchable to accommodate urine storage and voiding. Superficial umbrella cells express uroplakins (UPIA, UPII, UPIII), which are crucial for creating an impermeable membrane to prevent urine leakage and reabsorption of toxins. In vitro, HUCs have unique growth and differentiation characteristics. Primary HUCs, with their cobblestone-like appearance, grow in culture and require specific media (e.g., Keratinocyte-SFM or DMEM/F12 supplemented with growth factors).
Functionally, they are involved in mechanosensation (via TRP channels), immune signaling (e.g., IL-6/8 secretion), and regeneration (via basal stem cells). Their applications include bladder cancer research, uropathogen infection models, drug toxicity testing, and tissue engineering for bladder reconstruction. Challenges involve limited primary cell availability and maintaining in vivo-like properties in vitro. Advancements in 3D organoid culture and single-cell genomics are improving their utility in disease modeling and regenerative medicine.
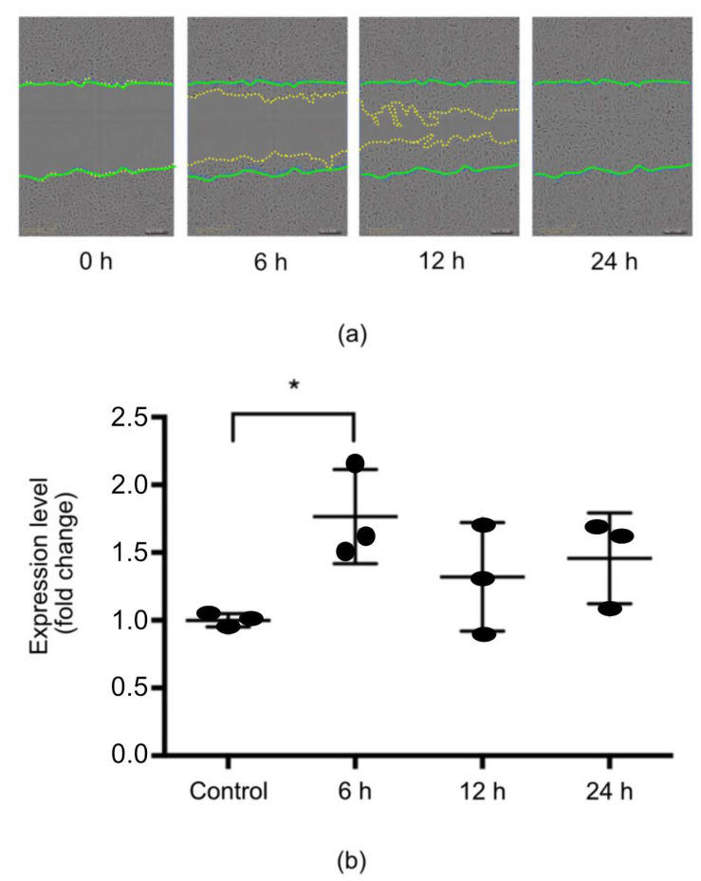
Expression of miR-132 in HUCs Was Regulated by Wound Healing Environment
Urinary bladder wound healing shares many features with skin healing, involving several molecular players, including microRNAs (miRs). Chamorro et al. investigated the role of miR-132 in urothelial cells.
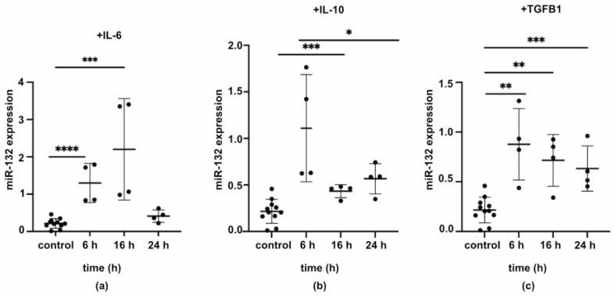
After in vitro scratch wounding, primary urothelial cells significantly increased miR-132 expression at 6 h. The levels of miR-132 were not significantly higher than control at 12 and 24 h (Fig. 1a). The wound was completely closed by 24 h (Fig. 1b). Thus, miR-132 is implicated in the early stages of epithelial response to wounding. To determine factors that modulate miR-132 expression during epithelial wound healing, urothelial cells were treated with IL-6, IL-10, and TGF-β1 (Fig. 2a–c), and miR-132 levels were examined at 6, 16, and 24 h. All of the cytokines upregulated miR-132 over time. IL-6 increased miR-132 levels at 6 and 16 h and returned to baseline by 24 h (Fig. 2a). IL-10 (Fig. 2b) and TGF-β1 (Fig. 2c) both continued to elevate miR-132 levels after 24 h. These results demonstrated that the expression of miR-132 was regulated by wound healing environment.
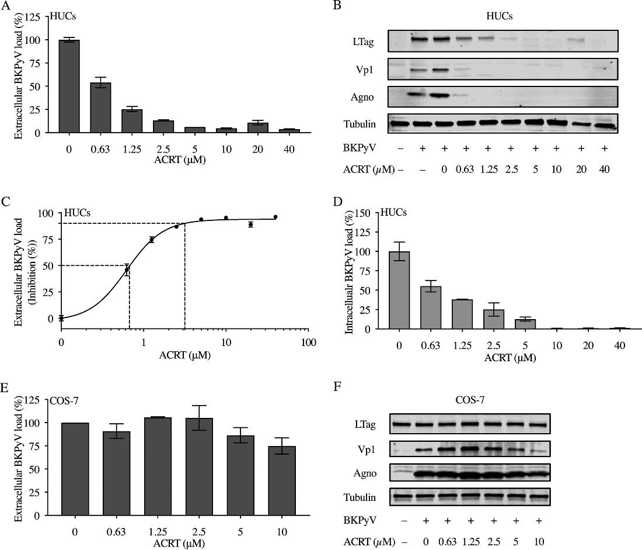
Acitretin inhibits BKPyV replication in primary HUCs but not in COS-7 cells.
Polyomavirus-associated nephropathy and hemorrhagic cystitis are complications in 5% to 25% of kidney and hematopoietic cell transplantations, highlighting the need for small-molecule drugs to inhibit BK polyomavirus (BKPyV).
Wu’s team investigated the inhibitory activity of acitretin (retinoic acid) against BKPyV replication in primary human renal proximal tubular epithelial cells (RPTECs) and urothelial cells. Acitretin inhibited BKPyV replication in primary human urothelial cells (HUCs) with an EC50 of 0.62 μM and EC90 of 3.12 μM, resulting in decreased viral protein expression and intracellular viral loads (Fig. 3A–D). Acitretin was also evaluated in COS-7 cells stably expressing SV40 LTag. The drug resulted in <25% inhibition even at the highest concentrations (Fig. 3E–F). There was no change in LTag levels; however, BKPyV-Vp1 and agnoprotein expression was reduced with 5 or 10 μM acitretin.
Ask a Question
Write your own review