C57BL/6 Mouse Uterine Epithelial Cells
Cat.No.: CSC-C9088J
Species: Mouse
Source: Uterus
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The uterine epithelial cells of C57BL/6 mice are derived from the endometrium. The endometrium is the inner layer of the uterine cavity, composed of a single layer of columnar epithelial cells. Its main function is related to the menstrual cycle and pregnancy. The function of endometrial epithelial cells is mainly reflected in hormone secretion and regulation of uterine environment, as well as the participation in embryo implantation and maintenance of pregnancy. They can express ERα and ERβ receptors in vitro and can be induced by growth factors and cytokines to regulate cell proliferation and differentiation.
Researchers widely use this cell type as an ideal model to examine numerous biological and medical phenomena. In reproductive biology, it can be used to study embryo implantation and endometrial receptivity. In endocrinology, it can be used to study the effects of hormones on the endometrium. In oncology, it can be used to establish an endometrial cancer model to explore the occurrence, development and metastasis of tumor in depth. In addition, it can be used to study cell-cell interaction, which is conducive to the exploration of intercellular signal and cell adhesion.
PROGERIN Overexpression Leads to Cytoplasmic DNA Accumulation and Cgas-STING Pathway Activation
As maternal age advances, fertility challenges such as reduced ovulation and lower pregnancy success become prevalent. Although ovarian aging's effects are known, uterine aging impacts remain debated, especially regarding uterine receptivity.
In this study, Chen's team used naturally aged one-year-old female mice to investigate effects of maternal age on embryo implantation during early pregnancy. They found abnormal uterine receptivity in aged mice. Aged mouse uterus indicates a decrease in nuclear LAMIN A, and an increase in PRELAMIN A and PROGERIN. In aged mouse uterus, double-stranded DNA (dsDNA) in cytoplasmic fraction is significantly increased. To investigate PROGERIN's role in aged mice's uterine cells, they transfected PROGERIN plasmids into mouse uterine epithelial cells, resulting in a marked increase in Progerin mRNA (Fig. 1a). PICOGREEN assays indicated a significant DNA concentration rise in the medium from these cells (Fig. 1b). The nuclear DNA (β-globin) in the cytoplasm of these cells was significantly elevated, but mitochondrial DNA levels (16S, Nd4, Dloop) remained unchanged (Fig. 1c). These findings suggest PROGERIN induces nuclear DNA leakage and cytoplasmic buildup. In PROGERIN-overexpressed cells, cGAS, STING, P-TBK1, and P-IRF3 protein levels were notably raised (Fig. 1d). In epithelial organoids, uterine receptivity markers (COX2, P-STAT3, AREG) decreased, whereas MUC1 increased (Fig. 1e). Thus, increased PROGERIN accumulates cytosolic DNA, activating the cGAS-STING pathway.
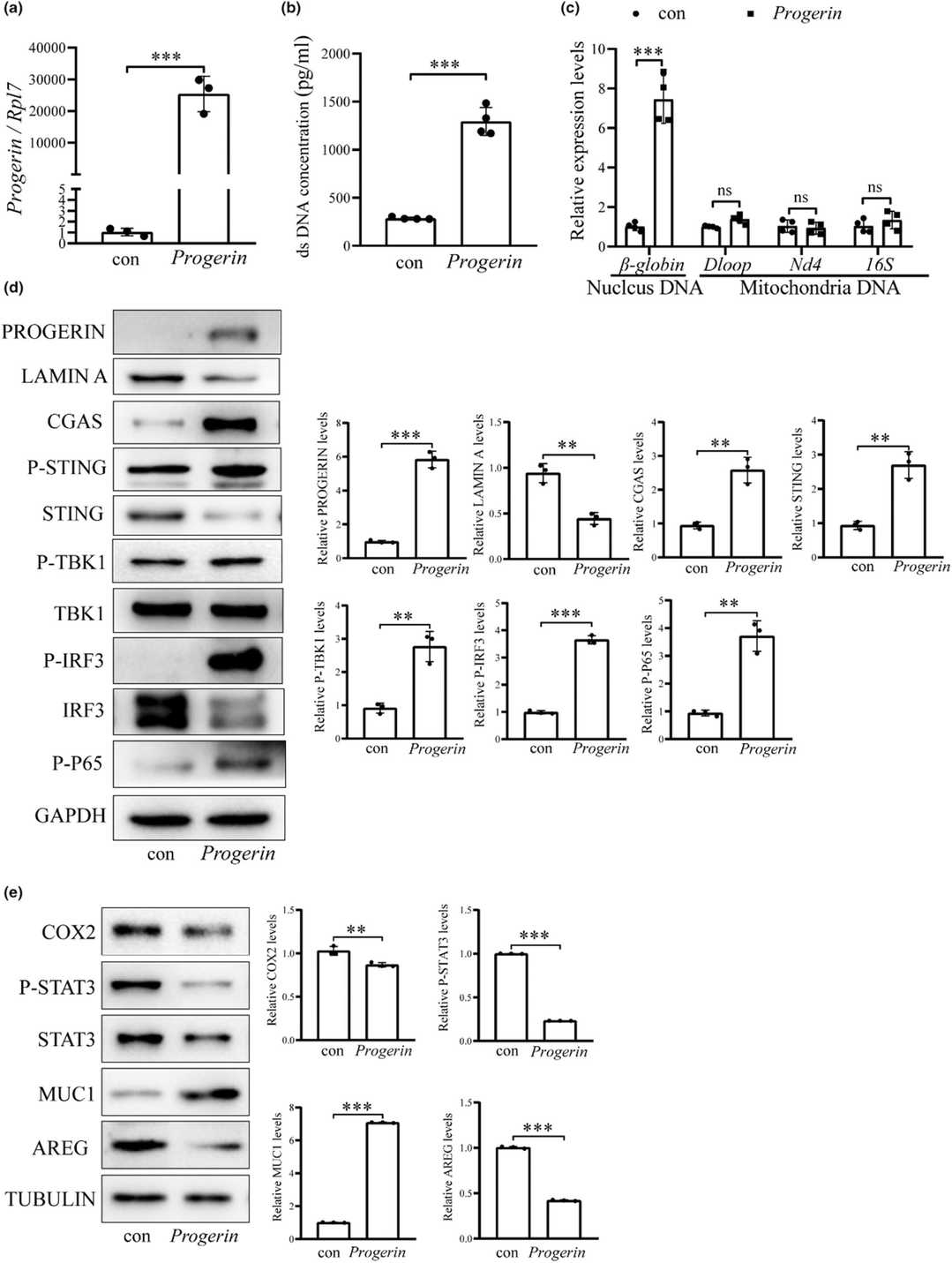 Fig. 1. PROGERIN overexpression leads to cytoplasmic DNA accumulation and cGAS-STING pathway activation (Chen S T, Shi W W, et al., 2024).
Fig. 1. PROGERIN overexpression leads to cytoplasmic DNA accumulation and cGAS-STING pathway activation (Chen S T, Shi W W, et al., 2024).
ATP-P2Y2 Signaling Increases the Phosphorylation of Stat3 in Uterine Epithelial Cells
Decidualization, essential for pregnancy, involves uterine remodeling for blastocyst implantation and relies on sterile inflammation mediated by ATP signaling. Gu's team explored ATP release during the peri-implantation period in mice, triggered by blastocyst-induced lactate release through connexin hemichannels.
Using in vivo experiments, they examined whether ATP-P2Y signaling was involved in embryo implantation. In mice, the P2Y receptors (P2YRs) include P2y1, P2Y2, P2y4, P2y6, P2y13, and P2y14. To identify which P2YR is essential for embryo implantation, we analyzed the transcript levels of these receptors in primary uterine epithelial cells from estrous mice. RT-PCR revealed that P2Y2 was most abundantly expressed, followed by P2y6, P2y14, P2y1, P2y13, and P2y4 (Fig. 2 A and B). This aligns with earlier findings of P2Y2 presence in rat uterine epithelium during early pregnancy. Immunofluorescence showed abundant P2Y2 in luminal and glandular epithelial cells from D1 to D4, increasing in stromal cells on D3 and D4 (Fig. 2C). Strong P2Y2 expression was also in the intestinal epithelium. Injecting the P2Y2 antagonist AR-C118925 into one uterine horn on D4 reduced implantation sites on D5 compared to the control (Fig. 2D and E). These findings suggest P2Y2 in uterine epithelium is crucial for embryo attachment and implantation.
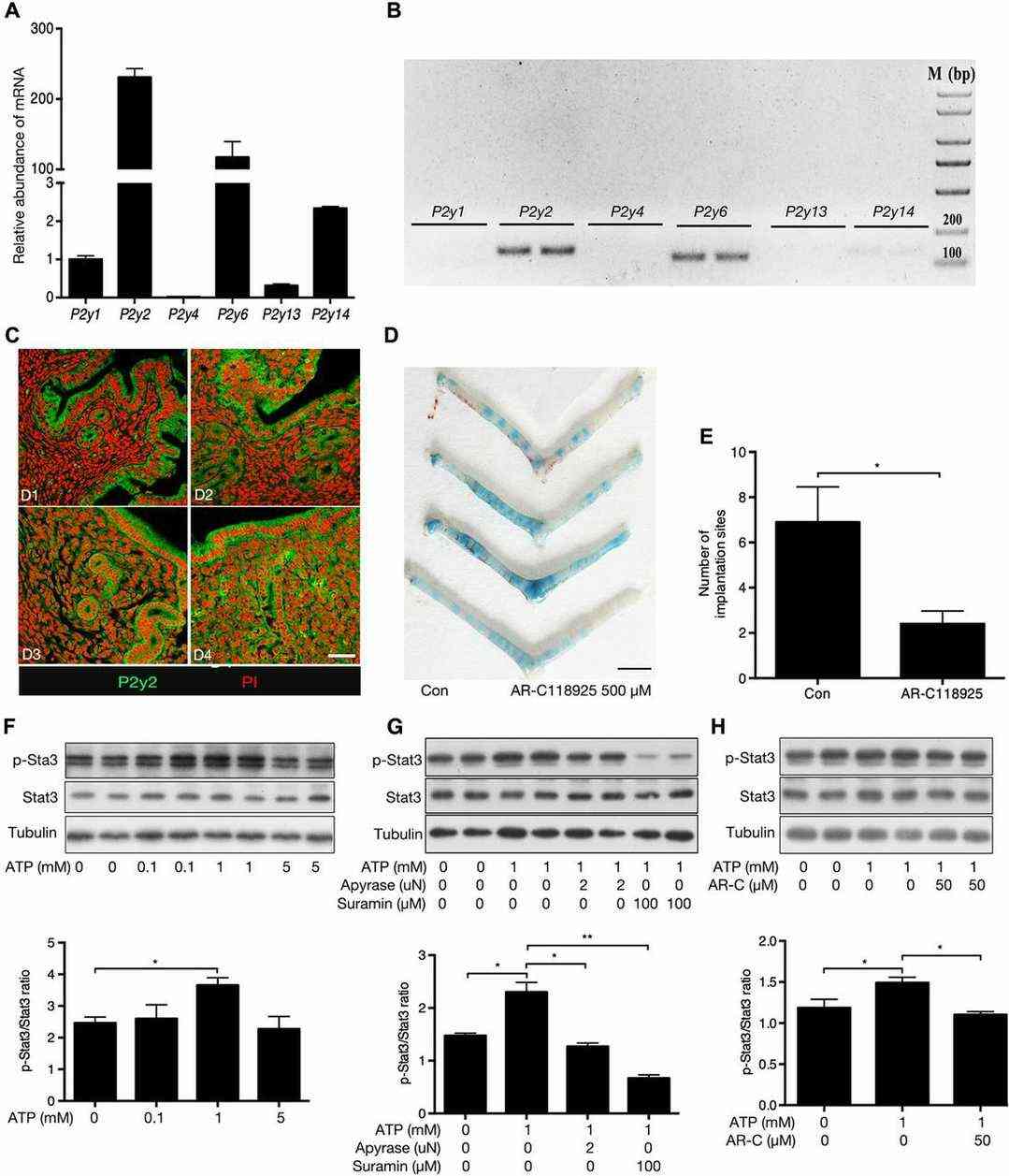 Fig. 2. P2Y2 signaling promotes implantation and Stat3 activation in uterine luminal epithelial cells (Gu X W, Chen Z C, et al., 2020).
Fig. 2. P2Y2 signaling promotes implantation and Stat3 activation in uterine luminal epithelial cells (Gu X W, Chen Z C, et al., 2020).
Ask a Question
Write your own review