C57BL/6 Mouse Small Intestinal Epithelial Cells
Cat.No.: CSC-C9085J
Species: Mouse
Source: Small Intestine; Intestine
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The small intestine is a crucial component of the digestive system, consisting of the duodenum, jejunum, and ileum. C57BL/6 mouse intestinal epithelial cells are the epithelial cells that line the inside of the small intestine. This epithelial tissue forms a protective layer on the inner surface of the small intestine and has functions such as nutrient absorption, mucus secretion, and immune defense. As the primary site for nutrient absorption, they effectively take up glucose, amino acids, and fatty acids. Additionally, these cells can secrete a variety of bioactive substances, such as mucus, digestive enzymes, and immune factors, contributing to gut health.
This cell line has significant applications in disease model research, allowing for the exploration of the pathogenesis and treatment methods for intestinal diseases such as Crohn's disease and ulcerative colitis. It also provides an ideal model for the study of the characteristics, differentiation, and regeneration mechanism of intestinal stem cells. In addition, the can be used for drug screening and toxicity testing, which can help researchers evaluate the impact of drugs on intestinal epithelial cells and aid in the development of safer and more effective drugs. Finally, this cell line can be used to study the interaction between gut microbiota and host epithelial cells, which can help researchers better understand how microorganisms regulate gene expression, metabolism, and immune responses in epithelial cells.
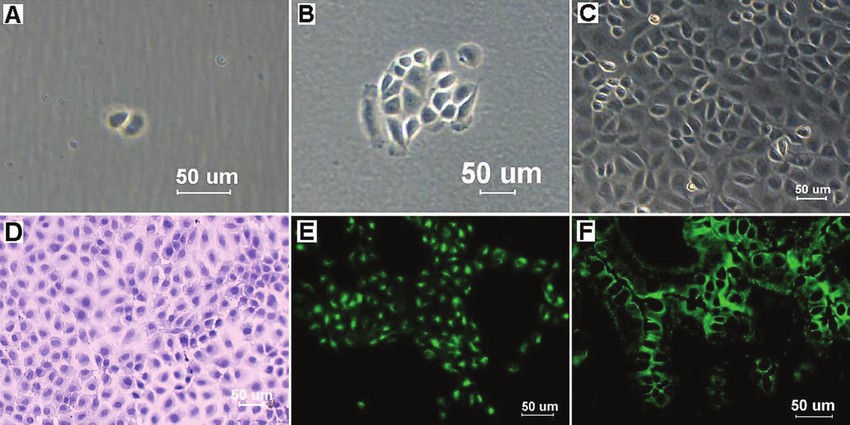 Fig. 1. Mouse IEC clones were obtained by limiting dilution, and light micrographs showed cell clones at 2 days (A) and 5 days (B) after seeding. The morphology of mouse IECs at the 35th passage is shown in (C). Hematoxylin and eosin staining showed that mouse IECs formed a cobblestone monolayer, and each cell was polygonal and flattened with a large, oval nucleus (D). The mouse IECs were strongly positive for cytokeratin 18 (E), and the mouse intestinal tissues also reacted with the cytokeratin 18 antibodies as the positive control (F) (Ren J H, Zhang C L, et al., 2017).
Fig. 1. Mouse IEC clones were obtained by limiting dilution, and light micrographs showed cell clones at 2 days (A) and 5 days (B) after seeding. The morphology of mouse IECs at the 35th passage is shown in (C). Hematoxylin and eosin staining showed that mouse IECs formed a cobblestone monolayer, and each cell was polygonal and flattened with a large, oval nucleus (D). The mouse IECs were strongly positive for cytokeratin 18 (E), and the mouse intestinal tissues also reacted with the cytokeratin 18 antibodies as the positive control (F) (Ren J H, Zhang C L, et al., 2017).
α-Chaconine Affects the Apoptosis Ability of Mouse Small Intestinal Epithelial Cells
Potato is a commonly used crop. However, the glycoalkaloids in potato are highly toxic to the digestive tract. α-Chaconine is the most abundant glycoalkaloid and is heat resistant and difficult to remove. Previous studies have reported that α-chaconine is cytotoxic, but the impact of α-chaconine on intestinal epithelial cells has not been fully reported.
He's team investigated how α-chaconine affects mouse small intestinal epithelial cells by evaluating its impact on proliferation, cell cycle, and apoptosis of intestinal epithelial cells at the cellular and molecular levels. α-Chaconine concentration and incubation time significantly affected the proliferation, cell cycle, and apoptosis of mouse intestinal epithelial cells (Fig. 1). Cell proliferation was significantly reduced with time in the 0.4 and 0.8 μg/mL groups and with increased concentration of α-chaconine at each time point. The ratio of G0/G1 phase increased with time and the concentration of α-chaconine. The proportion of S phase reduced by 10% at 48 h compared to 24 h in the control group, with no significant decrease at 72 h. In the 0.4 and 0.8 μg/mL groups, the S phase ratio decreased with time in a similar pattern to the control group, with an average decrease of 13% at 48 h and 24% at 72 h. In the control group, the G2/M phase ratio first increased and then decreased with time. In the 0.4 μg/mL group, the G2/M phase ratio did not change at 24 and 48 h and decreased at 72 h. In the 0.8 μg/mL group, the G2/M phase ratio showed a continuous decrease with time. At 24 h, α-chaconine had no significant effect on the G2/M phase ratio. At 48 and 72 h, the ratio decreased with an increase in concentration. The apoptotic cells increased with time in all groups and was positively correlated with the concentration of α-chaconine at each time point.
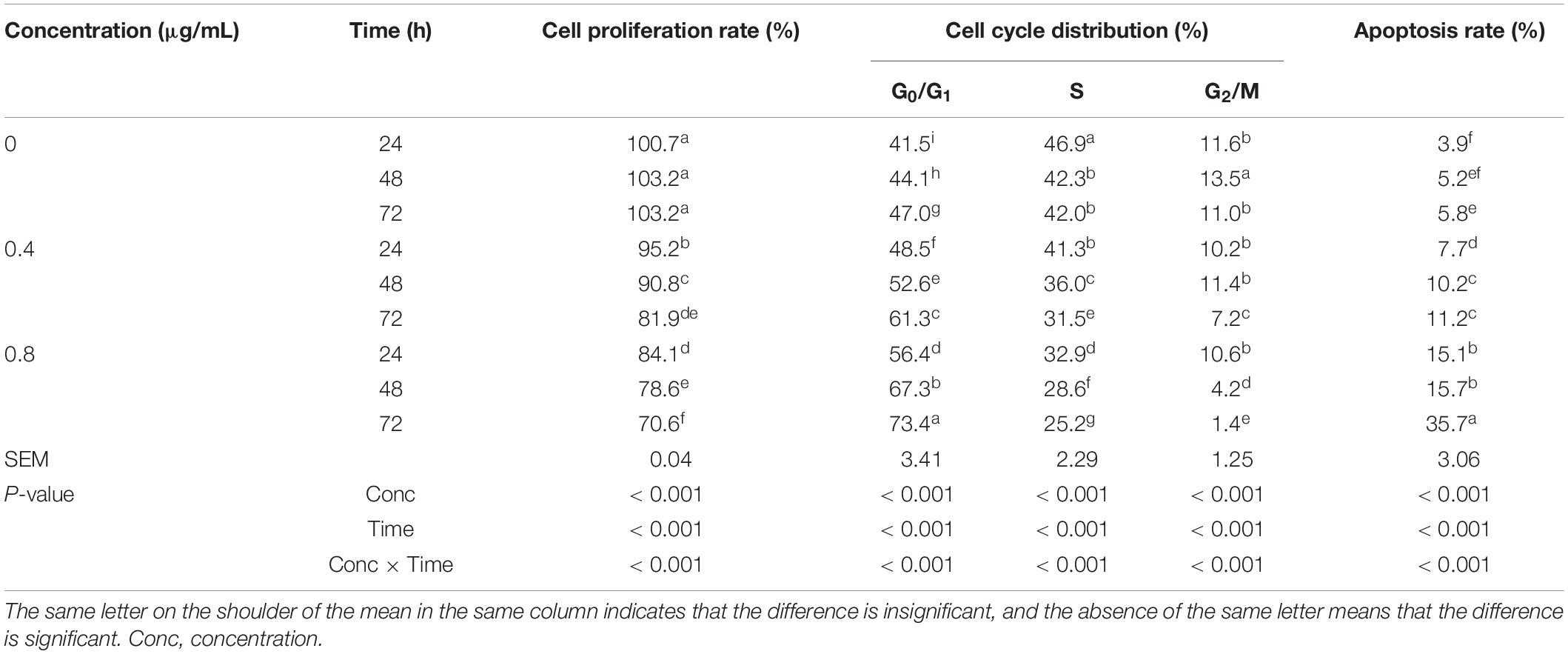 Fig. 1. Effects of α-chaconine on the proliferation, cell cycle, and apoptosis of mouse intestinal epithelial cells (He Y Y, Chen J Q, et al., 2021).
Fig. 1. Effects of α-chaconine on the proliferation, cell cycle, and apoptosis of mouse intestinal epithelial cells (He Y Y, Chen J Q, et al., 2021).
Propionate Enhances Cell Speed and Persistence to Promote Intestinal Epithelial Turnover and Repair
Short-chain fatty acids (SCFAs) are microbial metabolites that are necessary to maintain intestinal homeostasis. Although their effects on the regulation of intestinal immune function and barrier are being elucidated, their role in epithelial turnover and tissue repair remains to be determined. In this study, Bilotta et al. explored the unique role of propionate, a major SCFA, in the regulation of intestinal epithelial cell migration, as well as the precise contribution of propionate to epithelial renewal and mucosal wound healing following injury and inflammation.
To assess whether propionate promotes epithelial migration, they used an in vitro wound healing assay with mouse small intestinal epithelial cells (MSIEs). Propionate significantly enhanced cell migration into the denuded area over 20 hours (Fig. 2A, B), similar to acetate and butyrate. Similar migration effects were observed with both acetate and butyrate. MSIEs were then treated with SCFAs and stained for Ki67 to determine if the effect was a result of proliferation. They did not observe any difference in Ki67 expression (Fig. 2C, D). To further rule out a proliferation effect, the CFSE labeled cells and tracked their divisions over the course of the migration assay. There was no difference in the number of divisions between FBS treated and control cells (Fig. 2E, F). These data demonstrate that migration, and not proliferation, is the cause of the wound closure.
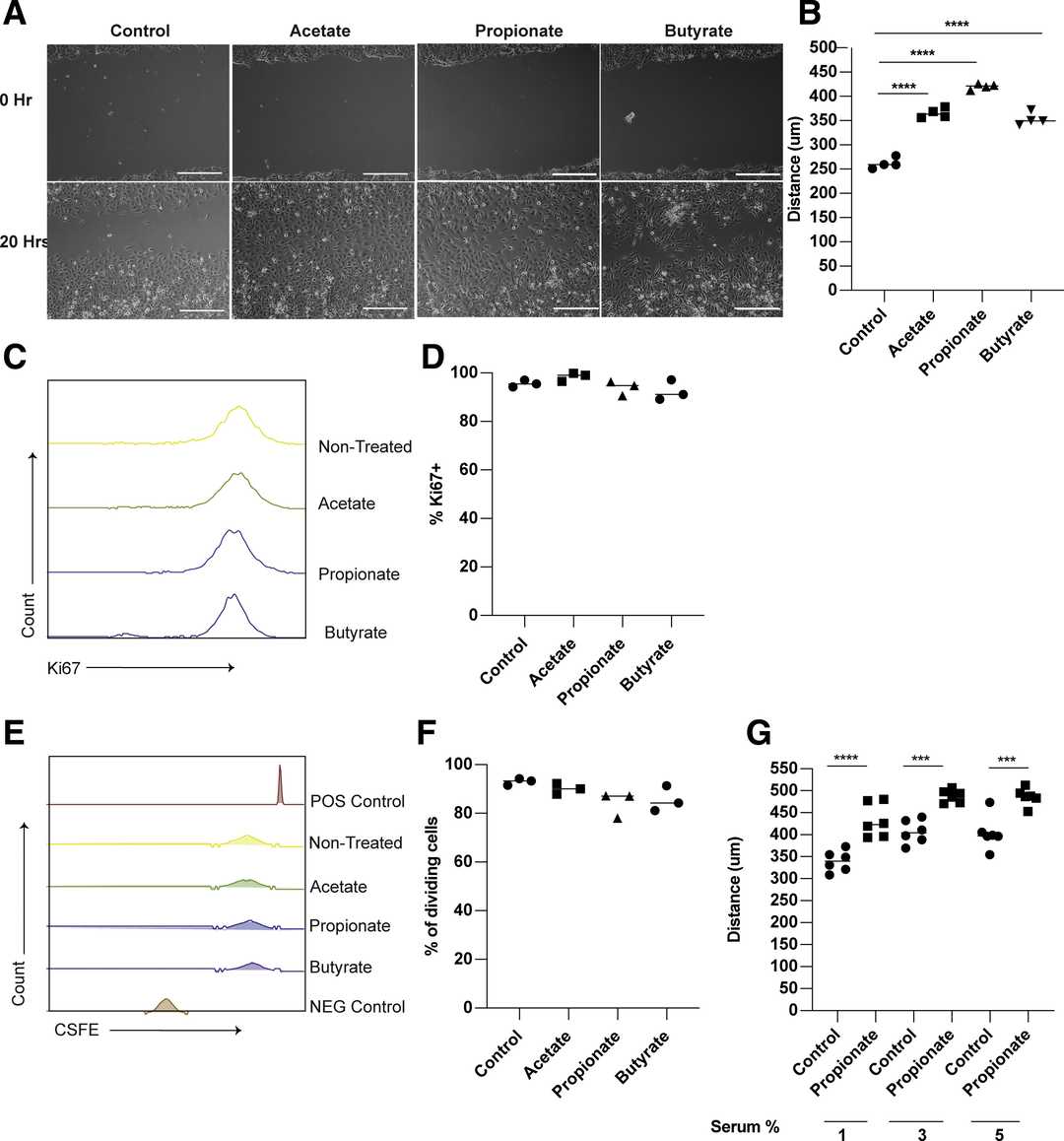 Fig. 2. Propionate promotes the migration of intestinal epithelial cells (Bilotta A J, Ma C Y, et al., 2020).
Fig. 2. Propionate promotes the migration of intestinal epithelial cells (Bilotta A J, Ma C Y, et al., 2020).
Ask a Question
Write your own review