C57BL/6 Mouse Primary Cardiac Fibroblasts
Cat.No.: CSC-C1878
Species: Mouse
Source: Heart
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Primary Cardiac Fibroblasts are negative for bacteria, yeast, fungi, and mycoplasma. Cells are tested for expression of marker using the antibody of anti-FSP1/S100A4 by immunofluorescence staining. Cells can be expanded for 3-5 passages at a split ratio of 1:2 under the cell culture conditions specified by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.Standard biochemical procedures performed with cell cultures include the assay of cell to cell interaction, RT-PCR, Western blotting, immunoprecipitation, immunofluorescent staining, flow cytometry or generating cell derivatives for desired research applications.
Primary cardiac fibroblasts from the common C57BL/6 mouse strain, a primary cell line, are non-immortalized and have limited lifespan. This in vitro model mimics the physiological conditions of cardiac fibroblasts in vivo. Primary cardiac fibroblasts are defined by their substantial secretion of ECM molecules, predominantly collagen, which is critical for structural support and homeostasis in the heart in the quiescent state. In conditions of cardiac injury, stress, or pro-fibrotic stimulation such as myocardial infarction (MI) or pressure overload, they become activated and transdifferentiate into myofibroblasts. This activated phenotype is associated with increased proliferation, enhanced ECM production, and expression of alpha-smooth muscle actin (α-SMA), which causes tissue fibrosis and scar formation. Functionally, these fibroblasts not only provide mechanical support but also play a critical role in cardiac remodeling, repair, and paracrine signaling with cardiomyocytes and endothelial cells in the heart through cell-cell crosstalk.
Owing to this critical role, C57BL/6 mouse primary cardiac fibroblasts serve as an essential model system to study cardiac fibrosis, pathological cardiac remodeling in heart failure, and the development of various cardiomyopathies. They are used widely for studying pro-fibrotic and anti-fibrotic signaling pathways, drug screening of anti-fibrotic compounds, and understanding cell-cell crosstalk in the cardiac microenvironment for identifying novel therapeutic approaches.
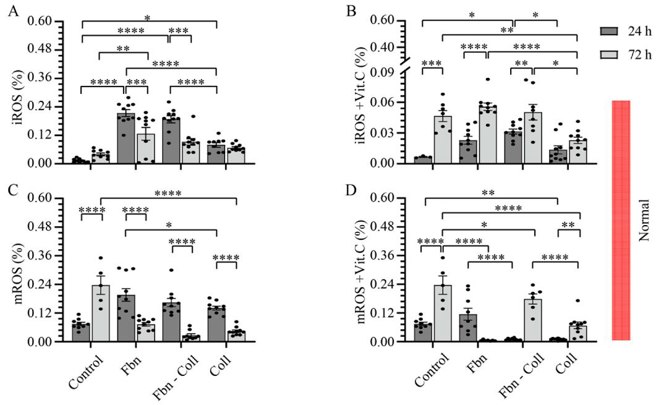
The Radical Scavenging Role of Vitamin C on Primary Cardiac Fibroblast
Post-MI healing depends on balanced collagen remodeling, yet regulators remain unclear. Xu's team asked whether vitamin C can steer cardiac fibroblast collagen production in a profibrotic milieu mimicking infarct repair. Mouse primary cardiac fibroblasts were isolated from wild-type C57BL/6 mice and cultured under normal and profibrotic (hypoxic + transforming growth factor beta 1) conditions on freshly prepared coatings mimicking extracellular matrix (ECM) remodeling during healing after an MI.
Experiments showed 10 µg/mL vitamin C scavenges radicals. Coatings mimic post-MI healing: fibronectin (early), fibronectin-collagen (mid), collagen (late). Under hypoxia + TGF-β1, ROS and mitochondrial ROS were quantified by DHE/mitoSOX and normalized to DAPI (Fig 1, 2). Without vitamin C, fibronectin raised cytosolic ROS to 0.23 ± 0.04 % at 24 h (Fig. 1A) and returned to control by 72 h. With vitamin C, values matched control, rising only slightly at 72 h (0.1 ± 0.06 %). Mitochondrial ROS peaked early then fell; at 72 h fibronectin suppressed it, whereas collagen coatings sustained elevation (Fig. 1C-D).

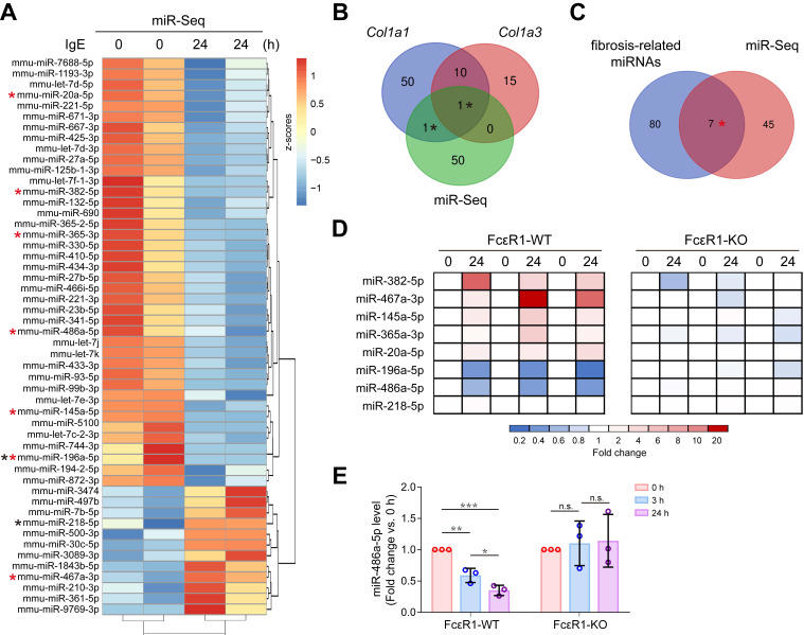
IgE Down-Regulated miR-486a-5p in Primary Cardiac Fibroblast
High IgE is linked to heart-failure fibrosis, yet how it acts on cardiac fibroblasts (CFs) is unknown. Zhao's team aims to clarify the IgE-FcεR1 pathway driving collagen production and identify microRNA mediators amenable to therapeutic correction.
To determine whether IgE drives cardiac fibrosis via miRNA remodeling, they performed miRNA-seq on IgE-treated CFs. Fifty-two IgE-responsive miRNAs were identified (heatmap, Fig. 3A); two of them-miR-196a-5p and miR-218-5p-were predicted by TargetScan7.1 to target Col1a1/Col3a1 (Fig. 3B). By cross-referencing our data with published fibrosis-miRNA sets, seven additional candidates emerged (miR-382-5p, -467a-3p, -145a-5p, -365-3p, -20a-5p, -196a-5p, -486a-5p; Venn, Fig. 3C). qPCR validation in FcεR1-WT vs FcεR1-KO CFs (Fig. 3D) confirmed that IgE significantly up-regulated miR-467a-3p and down-regulated miR-196a-5p and miR-486a-5p only when FcεR1 was present, matching the sequencing trend. Among these, miR-486a-5p exhibited the highest basal level; its expression declined time-dependently after IgE stimulation in WT but not KO cells (Fig. 3E). Thus, IgE-FcεR1 signaling selectively suppresses miR-486a-5p, implicating this miRNA in IgE-mediated cardiac fibrosis.
Ask a Question
Write your own review